Non-viral vectors for chimeric antigen receptor immunotherapy
IF 56
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
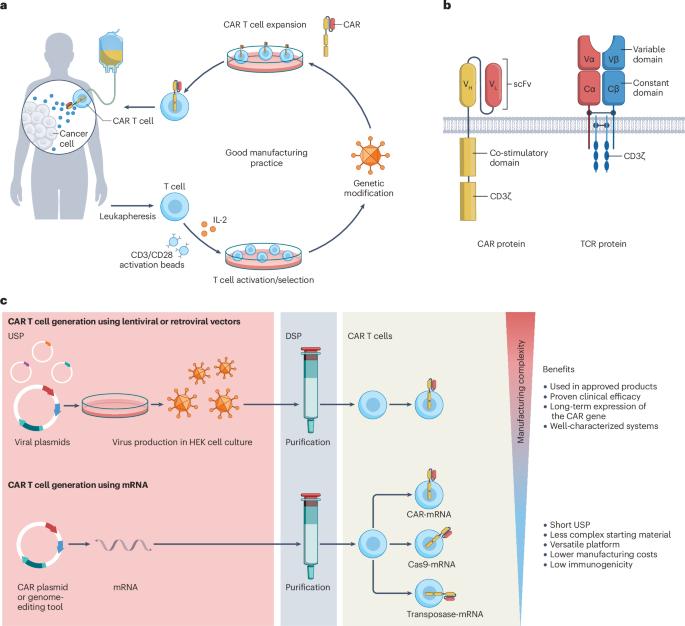
Chimeric antigen receptor (CAR) T cell therapy has demonstrated remarkable efficacy in haematological malignancies. However, although viral vector-based methods are effective for CAR T cell manufacturing, they present significant biological, logistical and financial challenges. Non-viral vectors — such as mRNA, transposon and precision genome editors — offer a promising alternative, addressing issues of scalability and cost. mRNA and precision genome-editing technologies hold promise in facilitating the implementation of safe in vivo gene delivery for CAR T cell therapy. In this Primer, we discuss recent advancements in non-viral vectors for CAR immunotherapy, focusing on methodologies, clinical results, opportunities and limitations. We discuss permanent and transient gene engineering technologies, highlighting their ability to efficiently deliver genetic material and perform multiple genetic edits in target effector cells, such as T lymphocytes. We briefly describe delivery methods and preclinical development of ex vivo and in vivo T cell engineering using non-viral vectors. Additionally, we outline key considerations for clinical translation, such as reproducibility and good manufacturing practice. Through a comprehensive review of current literature and clinical trials, we underscore the potential of non-viral vectors to further optimize CAR immunotherapies, paving the way for more advanced and accessible cell-based treatments. Non-viral vectors offer scalable, cost-effective alternatives for chimeric antigen receptor T cell therapy. In this Primer, Tretbar et al. highlight advances in the use of non-viral approaches, gene delivery methods, clinical outcomes and key considerations for translating these technologies into practice.
用于嵌合抗原受体免疫疗法的非病毒载体
嵌合抗原受体(CAR)T 细胞疗法已在血液恶性肿瘤中显示出显著疗效。然而,尽管基于病毒载体的方法对 CAR T 细胞的制造很有效,但它们在生物学、物流和财务方面都存在重大挑战。非病毒载体--如 mRNA、转座子和精准基因组编辑器--提供了一种有前途的替代方法,解决了可扩展性和成本问题。在本入门指南中,我们将讨论用于 CAR 免疫疗法的非病毒载体的最新进展,重点关注方法、临床结果、机遇和局限性。我们讨论了永久性和瞬时性基因工程技术,强调了这些技术在靶效应细胞(如 T 淋巴细胞)中有效递送遗传物质和进行多重基因编辑的能力。我们简要介绍了使用非病毒载体进行体内外 T 细胞工程的传递方法和临床前开发。此外,我们还概述了临床转化的关键注意事项,如可重复性和良好生产规范。通过对当前文献和临床试验的全面回顾,我们强调了非病毒载体进一步优化 CAR 免疫疗法的潜力,为更先进、更易获得的细胞疗法铺平了道路。非病毒载体为嵌合抗原受体 T 细胞疗法提供了可扩展、高成本效益的替代方案。在这本《入门》中,Tretbar 等人重点介绍了非病毒方法的使用进展、基因递送方法、临床结果以及将这些技术转化为实践的关键注意事项。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


