Repotrectinib: Redefining the therapeutic landscape for patients with ROS1 fusion-driven non-small cell lung cancer
Abstract
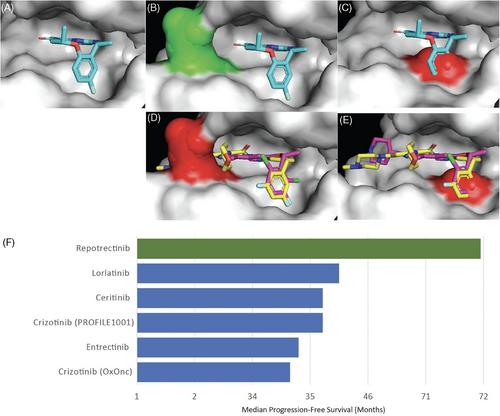
The ROS1 proto-oncogene encodes a receptor tyrosine kinase with structural homology to other oncogenic drivers, including ALK and TRKA-B-C. The FDA-approved tyrosine kinase inhibitors (TKIs) crizotinib and entrectinib have demonstrated efficacy in treating ROS1 fusion-positive NSCLC. However, limitations such as poor blood-brain barrier penetration and acquired resistance, particularly the ROS1 G2032R solvent-front mutation, hinder treatment durability. Repotrectinib, a next-generation macrocyclic TKI, was rationally designed to overcome on-target resistance mutations and improve brain distribution through its low molecular weight. In the TRIDENT-1 clinical trial, repotrectinib demonstrated significant efficacy in both TKI-naïve and TKI-pretreated patients with ROS1-rearranged NSCLC, including those with CNS metastases and G2032R resistance mutations. In the TKI-naïve cohort (n = 71), 79% of patients achieved an objective response, with a median progression-free survival (PFS) of 35.7 months, surpassing all previously approved ROS1 TKIs. In patients who had received one prior ROS1 TKI but were chemotherapy-naïve (n = 56), objective responses were observed in 38%, and median PFS was 9.0 months. The safety profile of repotrectinib was consistent with earlier-generation ROS1 TKIs and common adverse events included anemia, neurotoxicity, increased creatine kinase levels, and weight gain. These findings underscore the potential of repotrectinib to address unmet needs in ROS1-rearranged NSCLC, offering durable responses and improved intracranial activity. Future research should prioritize developing next-generation, selective ROS1 inhibitors to reduce Trk-mediated toxicities and improve treatment tolerance.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


