One-Pot Synthesis of 1,3-Diketones from Alkynones with the Assistance of Imidazole
IF 0.9
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
An efficient one-pot synthesis of 1,3-diketones has been synthesized starting from alkynones using a Michael addition–nucleophilic substitution reaction process. The reaction starts with the Michael addition of imidazoles to alkynones, followed by the nucleophilic substitution by water. The final product 1,3-diketones were characterized by the NMR spectra. This study demonstrated that such reactions could successfully occur in one pot under mild conditions with a high yield of 1,3-diketones from 65 to 93%.
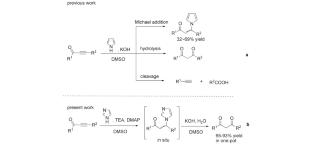
在咪唑的帮助下以炔酮为原料一步合成 1,3-二酮
我们利用迈克尔加成-亲核取代反应过程,从炔酮类化合物开始,高效地一步合成了 1,3-二酮。该反应首先是咪唑与炔酮发生迈克尔加成反应,然后用水进行亲核取代。通过核磁共振光谱对最终产物 1,3-二酮进行了表征。该研究表明,此类反应可在温和的条件下一锅成功完成,1,3-二酮的收率高达 65% 至 93%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


