High circulating activin A plasma levels are associated with tumour stage and poor survival in treatment-naive lung squamous cell cancer patients
IF 5
2区 医学
Q2 Medicine
引用次数: 0
Abstract
Objectives
Lung squamous cell carcinoma (LUSC) is associated with a poor prognosis and a lack of specific treatment options. The dysregulation of activin A (ActA) has been reported in various malignancies. Herein, we investigated the diagnostic and prognostic significance of ActA in LUSC.
Materials and methods
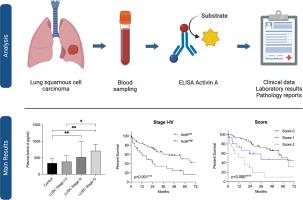
ActA concentrations were measured using ELISA in plasma samples of 128 LUSC patients (stage I-IV) and 73 controls, and correlated those values with clinicopathological parameters and survival.
Results
ActA plasma levels were significantly higher in therapy-naive LUSC patients compared to controls (444.1 310.9 pg/mL vs 338.9 145.5 pg/mL, p = 0.010). ActA levels significantly correlated with advanced stage as well as with T and N factors. High circulating ActA levels were significantly increased in metastatic disease patients compared to M0 disease. Further, patients with ActA levels above a computationally established optimal cut-off value of 443.0 pg/mL had a significantly worse median overall (OS, 17.63 vs 64.77 months, HR 0.391, 95 % CI 0.200–0.762, p < 0.001) and median disease-/progression-free survival (DFS/PFS; 11.57 vs 30.20 months, HR 0.502, 95 % CI 0.248–1.019, p = 0.020). Multivariate analysis revealed that high ActA levels were an independent prognostic factor for shorter OS (p = 0.001) and DFS/PFS (p = 0.018). A newly developed score combining CRP and ActA levels was also an independent prognostic factor for OS and DFS/PFS.
Conclusion
Measurement of circulating ActA levels may help identify advanced-stage LUSC patients, and this value could serve as a prognostic parameter in LUSC. Thus, ActA may be a novel blood-based biomarker for identifying LUSC patients with distant metastasis.
循环激活素 A 血浆水平高与肿瘤分期和未经治疗的肺鳞癌患者生存率低有关
目的肺鳞状细胞癌(LUSC)预后不良,且缺乏特异性治疗方案。各种恶性肿瘤中都有活化素 A(ActA)失调的报道。在此,我们研究了 ActA 在 LUSC 中的诊断和预后意义。材料和方法使用酶联免疫吸附法测定了 128 例 LUSC 患者(I-IV 期)和 73 例对照者血浆样本中的 ActA 浓度,并将这些值与临床病理参数和存活率相关联。结果与对照者相比,未经治疗的 LUSC 患者的 ActA 血浆水平显著更高(444.1 ± 310.9 pg/mL vs 338.9 ± 145.5 pg/mL,p = 0.010)。ActA水平与晚期以及T和N因子有明显相关性。与 M0 疾病相比,高循环 ActA 水平在转移性疾病患者中明显增加。此外,ActA水平高于经计算确定的最佳临界值443.0 pg/mL的患者的中位总生存期(OS,17.63 vs 64.77个月,HR 0.391,95 % CI 0.200-0.762,p < 0.001)和中位无疾病/无进展生存期(DFS/PFS;11.57 vs 30.20个月,HR 0.502,95 % CI 0.248-1.019,p = 0.020)明显更差。多变量分析显示,高 ActA 水平是缩短 OS(p = 0.001)和 DFS/PFS(p = 0.018)的独立预后因素。结合 CRP 和 ActA 水平新开发的评分也是 OS 和 DFS/PFS 的独立预后因素。因此,ActA可能是一种新型的血液生物标记物,可用于鉴别有远处转移的LUSC患者。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Translational Oncology
ONCOLOGY-
CiteScore
8.40
自引率
2.00%
发文量
314
审稿时长
54 days
期刊介绍:
Translational Oncology publishes the results of novel research investigations which bridge the laboratory and clinical settings including risk assessment, cellular and molecular characterization, prevention, detection, diagnosis and treatment of human cancers with the overall goal of improving the clinical care of oncology patients. Translational Oncology will publish laboratory studies of novel therapeutic interventions as well as clinical trials which evaluate new treatment paradigms for cancer. Peer reviewed manuscript types include Original Reports, Reviews and Editorials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


