Methionine oxidation of actin cytoskeleton attenuates traumatic memory retention via reactivating dendritic spine morphogenesis
IF 10.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Post-traumatic stress disorder (PTSD) is characterized by hypermnesia of the trauma and a persistent fear response. The molecular mechanisms underlying the retention of traumatic memories remain largely unknown, which hinders the development of more effective treatments. Utilizing auditory fear conditioning, we demonstrate that a redox-dependent dynamic pathway for dendritic spine morphogenesis in the basolateral amygdala (BLA) is crucial for traumatic memory retention. Exposure to a fear-induced event markedly increased the reduction of oxidized filamentous actin (F-actin) and decreased the expression of the molecule interacting with CasL 1 (MICAL1), a methionine-oxidizing enzyme that directly oxidizes and depolymerizes F-actin, leading to cytoskeletal dynamic abnormalities in the BLA, which impairs dendritic spine morphogenesis and contributes to the persistence of fearful memories. Following fear conditioning, overexpression of MICAL1 in the BLA inhibited freezing behavior during fear memory retrieval via reactivating cytokinesis, whereas overexpression of methionine sulfoxide reductase B 1, a key enzyme that reduces oxidized F-actin monomer, increased freezing behavior during retrieval. Notably, intra-BLA injection of semaphorin 3A, an endogenous activator of MICAL1, rapidly disrupted fear memory within a short time window after conditioning. Collectively, our results indicate that redox modulation of actin cytoskeleton in the BLA is functionally linked to fear memory retention and PTSD-like memory.
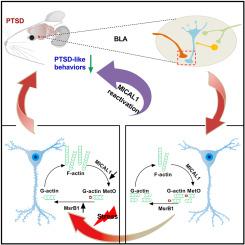
肌动蛋白细胞骨架的蛋氨酸氧化可通过重新激活树突棘形态发生减轻创伤记忆的保持
创伤后应激障碍(PTSD)的特点是对创伤记忆过度和持续的恐惧反应。创伤记忆保留的分子机制在很大程度上仍然未知,这阻碍了更有效治疗方法的开发。我们利用听觉恐惧条件反射证明,杏仁基底外侧(BLA)树突棘形态发生的氧化还原依赖性动态途径对创伤记忆的保留至关重要。暴露于恐惧诱导的事件明显增加了氧化丝状肌动蛋白(F-actin)的减少,并降低了与CasL 1相互作用的分子(MICAL1)的表达,MICAL1是一种蛋氨酸氧化酶,可直接氧化和解聚F-actin,从而导致杏仁基底节细胞骨架动态异常,这损害了树突棘形态发生,并导致恐惧记忆的持续。恐惧条件反射后,在BLA中过表达MICAL1可通过重新激活细胞分裂抑制恐惧记忆检索过程中的冻结行为,而过表达蛋氨酸亚砜还原酶B 1(一种减少氧化F-肌动蛋白单体的关键酶)则会增加检索过程中的冻结行为。值得注意的是,在BLA内注射内源性激活剂MICAL1的semaphorin 3A会在条件反射后的短时间内迅速破坏恐惧记忆。总之,我们的研究结果表明,BLA中肌动蛋白细胞骨架的氧化还原调节与恐惧记忆保持和创伤后应激障碍样记忆有功能上的联系。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


