Catalytic benzylation of arenes using metal-ion modified HY zeolites for sustainable synthesis
IF 4.3
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Microporous zeolites are commonly employed as catalysts for the benzylation of arenes and benzyl alcohol. However, their catalytic efficiency is often compromised by diffusion limitations, particularly in reactions involving larger arenes. In this study, we developed metal-ion modified HY zeolites using Zn, Mg, and Ni as dopants and investigated their catalytic performance in the benzylation of a range of arenes, including toluene, benzene, mesitylene, p-xylene and with benzyl alcohol (BzOH). The structural and acidic properties of the modified HY zeolites were characterized using a combination of techniques including scanning electron microscopy (SEM), X-ray diffraction (XRD), N₂ adsorption–desorption isotherms, Fourier-transform infrared (FTIR) spectroscopy, ammonia temperature-programmed desorption (NH₃-TPD), and proton magic angle spinning nuclear magnetic resonance (1H MAS NMR) spectroscopy. The presence of catalytically active Brønsted acid sites (BAS) was detected by 1H MAS NMR spectroscopy and it was demonstrated that the HY zeolite's acidity is considerably modulated by the addition of metal ions. The catalytic evaluations indicated that the metal-ion modified HY zeolites exhibited superior activity compared to unmodified HY zeolite, with the catalytic performance following the order Zn/HY > Ni/HY > Mg/HY > HY for the benzylation of benzyl alcohol with mesitylene. Further investigation into the mechanism revealed that the synergistic effect of metal ions and acidity plays a crucial role in enhancing the accessibility of arenes to the surface catalytic sites and thereby improving catalytic performance. These findings underscore the importance of the metal-acidity synergy in optimizing the catalytic efficacy of modified HY zeolites for selective benzylation reactions.
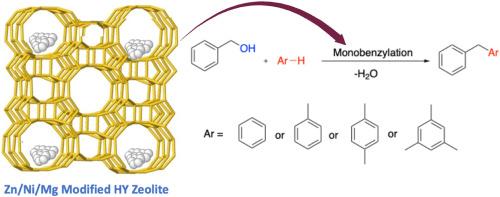
利用金属离子修饰的 HY 沸石催化炔烃的苄基化,实现可持续合成
微孔沸石通常用作茴香和苄醇苄基化反应的催化剂。然而,它们的催化效率往往会受到扩散限制的影响,尤其是在涉及较大的炔类化合物的反应中。在本研究中,我们开发了以 Zn、Mg 和 Ni 为掺杂剂的金属离子修饰 HY 沸石,并研究了它们在一系列烯烃(包括甲苯、苯、间二甲苯、对二甲苯和苯甲醇 (BzOH) )的苄化反应中的催化性能。利用扫描电子显微镜 (SEM)、X 射线衍射 (XRD)、N₂ 吸附-解吸等温线、傅立叶变换红外光谱 (FTIR)、氨温编程解吸 (NH₃-TPD) 和质子魔角旋转核磁共振 (1H MAS NMR) 光谱等多种技术对改性 HY 沸石的结构和酸性特性进行了表征。1H MAS NMR 光谱检测了催化活性布氏酸位点 (BAS) 的存在,并证明 HY 沸石的酸性受金属离子添加的影响很大。催化评估结果表明,与未改性的 HY 沸石相比,金属离子改性的 HY 沸石在苯甲醇与介二甲苯的苄基化反应中表现出更高的活性,催化性能依次为 Zn/HY > Ni/HY > Mg/HY > HY。对其机理的进一步研究表明,金属离子和酸性的协同作用在提高茴香对表面催化位点的可及性从而提高催化性能方面起着至关重要的作用。这些发现强调了金属酸性协同作用在优化改性 HY 沸石选择性苄基化反应催化效能方面的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Chemistry and Physics
工程技术-材料科学:综合
CiteScore
8.70
自引率
4.30%
发文量
1515
审稿时长
69 days
期刊介绍:
Materials Chemistry and Physics is devoted to short communications, full-length research papers and feature articles on interrelationships among structure, properties, processing and performance of materials. The Editors welcome manuscripts on thin films, surface and interface science, materials degradation and reliability, metallurgy, semiconductors and optoelectronic materials, fine ceramics, magnetics, superconductors, specialty polymers, nano-materials and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


