Vapor-liquid equilibria for the CO2 + trimethoxymethylsilane and CO2 + triethoxymethylsilane systems under high-pressure conditions
IF 2.8
3区 工程技术
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
New binary isotherms are crucial for designing chemical separation processes within supercritical carbon dioxide (CO2) + trialkoxysilane systems. Vapor-liquid equilibria (VLE) were investigated for two-component systems, trimethoxymethylsilane + CO2 and triethoxymethylsilane + CO2, at five temperatures (313.2, 333.2, 353.2, 373.2, and 393.2 K) and pressures up to 14.07 MPa using a synthetic high-pressure phase equilibria apparatus. The pressure-temperature (P-T) plot indicates that the critical mixture curve lies between the critical points of CO2 and the trialkoxysilane compounds. The solubility of trimethoxymethylsilane and triethoxymethylsilane in CO2 increased with increasing temperature at constant pressure, following a type-I phase behavior characteristic. The experimentally observed VLE values of the CO2 + trialkoxysilane systems were correlated using the Peng-Robinson equation of state with binary parameters (kij and ηij) in the conventional mixing rule. The model accuracy was validated by calculating the average relative deviation percentage for the pressure of the binary systems, resulting in values of 4.98% for the trimethoxymethylsilane + CO2 system and 3.64% for the triethoxymethylsilane + CO2 system. The estimated variables fell within reasonable limits and showed no significant differences between the predicted and observed VLE data for both systems.
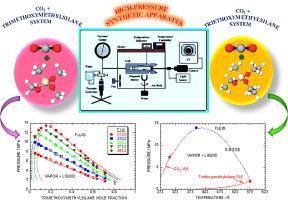
高压条件下二氧化碳+三甲氧基甲基硅烷和二氧化碳+三甲氧基甲基硅烷体系的气液平衡
新的二元等温线对于设计超临界二氧化碳(CO2)+三烷氧基硅烷体系中的化学分离过程至关重要。使用合成的高压相平衡仪器,在五种温度(313.2、333.2、353.2、373.2 和 393.2 K)和最高 14.07 MPa 的压力下,研究了双组分系统(三甲氧基甲基硅烷 + CO2 和三甲氧基甲基硅烷 + CO2)的汽液平衡 (VLE)。压力-温度(P-T)图表明,临界混合物曲线位于二氧化碳和三烷氧基硅烷化合物的临界点之间。在恒压条件下,三甲氧基甲基硅烷和三甲氧基甲基硅烷在 CO2 中的溶解度随着温度的升高而增加,这符合 I 型相行为特征。利用彭-罗宾逊状态方程和传统混合规则中的二元参数(kij 和 ηij),将实验观察到的 CO2 + 三烷氧基硅烷体系的 VLE 值联系起来。通过计算二元体系压力的平均相对偏差百分比验证了模型的准确性,结果是三甲氧基甲基硅烷 + CO2 体系的相对偏差百分比为 4.98%,三甲氧基甲基硅烷 + CO2 体系的相对偏差百分比为 3.64%。估算的变量均在合理范围内,且两种体系的预测 VLE 数据与观测 VLE 数据之间无明显差异。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fluid Phase Equilibria
工程技术-工程:化工
CiteScore
5.30
自引率
15.40%
发文量
223
审稿时长
53 days
期刊介绍:
Fluid Phase Equilibria publishes high-quality papers dealing with experimental, theoretical, and applied research related to equilibrium and transport properties of fluids, solids, and interfaces. Subjects of interest include physical/phase and chemical equilibria; equilibrium and nonequilibrium thermophysical properties; fundamental thermodynamic relations; and stability. The systems central to the journal include pure substances and mixtures of organic and inorganic materials, including polymers, biochemicals, and surfactants with sufficient characterization of composition and purity for the results to be reproduced. Alloys are of interest only when thermodynamic studies are included, purely material studies will not be considered. In all cases, authors are expected to provide physical or chemical interpretations of the results.
Experimental research can include measurements under all conditions of temperature, pressure, and composition, including critical and supercritical. Measurements are to be associated with systems and conditions of fundamental or applied interest, and may not be only a collection of routine data, such as physical property or solubility measurements at limited pressures and temperatures close to ambient, or surfactant studies focussed strictly on micellisation or micelle structure. Papers reporting common data must be accompanied by new physical insights and/or contemporary or new theory or techniques.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


