Dissolved calcium issues in estuaries and marine areas: Review of the Chinese Coast
IF 2.5
3区 地球科学
Q2 GEOSCIENCES, MULTIDISCIPLINARY
引用次数: 0
Abstract
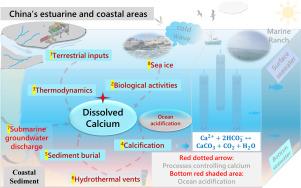
Dissolved calcium plays a critical role in coastal environments, influencing marine ecosystems and fisheries. This review comprehensively examines current research on dissolved calcium along China's coast. Despite its importance, research on dissolved calcium in China's estuarine and coastal areas is still in its early stages, spanning estuaries, continental shelf seas, and bays, yet with limited literature available. This review advocates for the use of automated EGTA (C14H24N2O10) potentiometric titration for accurate determination and quality control of dissolved calcium. Alternative techniques, such as ion chromatography and ICP-MS, are also viable options, while methods like fluorescence, capillary zone electrophoresis, and pulsed constant current control should be approached cautiously. Thermodynamics, terrestrial inputs, biological activity, and calcification influence dissolved calcium dynamics, resulting in its excess presence. Investigating the distribution and contributing processes of this excess calcium poses significant research challenges. Special scenarios including sea ice, submarine groundwater discharge, and hydrothermal vents warrant further investigation for their impact on excess calcium. Discrepancies between Ωarag calculated from measured dissolved calcium and carbonate (CO32−), and apparent solubility product (Ksp) versus calcium-salt ratios, may introduce inaccuracies in acidification assessments. The intricate nature of calcium ions and their geochemical implications should be carefully considered when studying coastal acidification effects on the calcium carbonate system. While focusing on China's coastal regions, insights from these studies could substantially contribute to global research and management of coastal acidification.
河口和海域的溶解钙问题:中国海岸回顾
溶解钙在沿岸环境中起着至关重要的作用,影响着海洋生态系统和渔业。本综述全面考察了中国沿海溶解钙的研究现状。尽管中国河口和沿海地区的溶解钙研究十分重要,但目前仍处于早期阶段,研究范围包括河口、大陆架海域和海湾,可获得的文献有限。本综述提倡使用自动 EGTA(C14H24N2O10)电位滴定法对溶解钙进行准确测定和质量控制。离子色谱法和 ICP-MS 等替代技术也是可行的选择,而荧光、毛细管区电泳和脉冲恒流控制等方法则应谨慎对待。热力学、陆地输入、生物活动和钙化都会影响溶解钙的动力学,导致钙过量存在。调查过量钙的分布和形成过程是一项重大的研究挑战。海冰、海底地下水排放和热液喷口等特殊情况对过量钙的影响值得进一步研究。根据测量到的溶解钙和碳酸盐(CO32-)计算出的Ωarag,以及表观溶度积(Ksp)与钙盐比值之间的差异,可能会给酸化评估带来误差。在研究沿岸酸化对碳酸钙系统的影响时,应仔细考虑钙离子的复杂性及其地球化学影响。虽然这些研究的重点是中国沿海地区,但它们对全球沿岸酸化的研究和管理具有重要意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Marine Systems
地学-地球科学综合
CiteScore
6.20
自引率
3.60%
发文量
81
审稿时长
6 months
期刊介绍:
The Journal of Marine Systems provides a medium for interdisciplinary exchange between physical, chemical and biological oceanographers and marine geologists. The journal welcomes original research papers and review articles. Preference will be given to interdisciplinary approaches to marine systems.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


