A DFT investigation of monolayer InP: Effective toxic gas sensor with adsorption and optical response
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
An examination was conducted on the electronic and optical characteristics of gas (H2S, CO2, CO, SO2, and SO) adsorption on a monolayer of InP using first-principles calculations based on DFT. To identify the optimal and most sensitive adsorption site for the adsorbed gases, four initial adsorption sites were selected. Various aspects such as adsorption distance, charge densities, and adsorption energy were analyzed across different types of adsorption to determine the most favorable adsorption configurations. Our research indicates that InP monolayers can chemically adsorb CO2, CO, SO2, and SO, forming new bonds with these gas molecules. Furthermore, H2S can be physically absorbed onto InP with a high level of adsorption energy. The optical findings reveal that the presence of gas molecules alters the conductivity and optical properties of the InP monolayer, especially noticeable in the UV range. InP emerges as a suitable material for detecting CO2, CO, SO2, and SO due to its distinct characteristics.
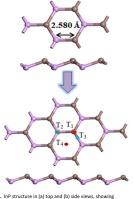
单层 InP 的 DFT 研究:具有吸附和光学响应的有效有毒气体传感器
利用基于 DFT 的第一性原理计算,研究了气体(H2S、CO2、CO、SO2 和 SO)在 InP 单层上吸附的电子和光学特性。为了确定被吸附气体的最佳和最敏感吸附位点,我们选择了四个初始吸附位点。我们分析了不同吸附类型的吸附距离、电荷密度和吸附能量等各个方面,以确定最有利的吸附配置。我们的研究表明,InP 单层可以化学吸附 CO2、CO、SO2 和 SO,并与这些气体分子形成新的键。此外,InP 还能以高吸附能吸附 H2S。光学研究结果表明,气体分子的存在改变了 InP 单层的导电性和光学特性,在紫外线范围内尤为明显。InP 因其独特的特性而成为检测 CO2、CO、SO2 和 SO 的合适材料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


