Pyrolysis reaction mechanisms of nitroglycerin (NG) and 1,2,4-butanetriol trinitrate (BTTN) using the ReaxFF force field
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
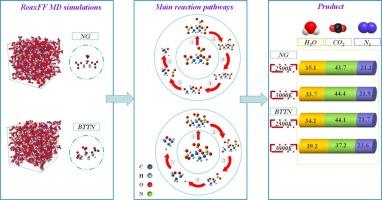
In this study, the ReaxFF-lg reactive force field was verified and employed to simulate the thermal decomposition of nitroglycerin (NG) and 1,2,4-butanetriol trinitrate (BTTN) at various temperatures (1500–3000 K), elucidating the detailed mechanisms of initial changes, intermediate products, and final product reactions for both energetic materials. The results indicate that the initial reactions of NG and BTTN are similar, and the primary reactions involve the cleavage of the O-NO2 bond and the dissociation of the C![]() C bond. At 1500 K, the breakage of O-NO2 bonds becomes the dominant reaction. As the temperature increases, in addition to the removal of NO2, the breakage of C
C bond. At 1500 K, the breakage of O-NO2 bonds becomes the dominant reaction. As the temperature increases, in addition to the removal of NO2, the breakage of C![]() C chains is observed above 2000 K. The formation of intermediates NO2, HNO2, NO, and CH2O accelerates the chemical reaction. The proportions of CO2 and H2O in the final products of BTTN change significantly with temperature.
C chains is observed above 2000 K. The formation of intermediates NO2, HNO2, NO, and CH2O accelerates the chemical reaction. The proportions of CO2 and H2O in the final products of BTTN change significantly with temperature.
利用 ReaxFF 力场分析硝化甘油(NG)和 1,2,4-丁三醇三硝酸酯(BTTN)的热解反应机理
本研究验证了 ReaxFF-lg 反应力场,并利用该力场模拟了硝化甘油(NG)和 1,2,4-丁三醇三硝酸酯(BTTN)在不同温度(1500-3000 K)下的热分解过程,阐明了这两种高能物质的初始变化、中间产物和最终产物反应的详细机理。结果表明,NG 和 BTTN 的初始反应相似,主要反应涉及 O-NO2 键的裂解和 CC 键的解离。在 1500 K 时,O-NO2 键的断裂成为主要反应。中间产物 NO2、HNO2、NO 和 CH2O 的形成加速了化学反应。BTTN 最终产物中 CO2 和 H2O 的比例随温度的升高而发生显著变化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


