Conformational analysis of the IQSEC2 protein by statistical thermodynamics
IF 2.7
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
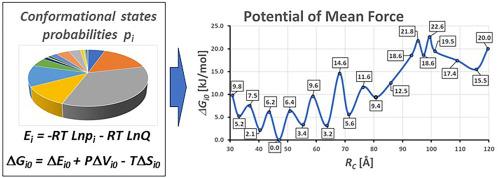
Mutations in the IQSEC2 gene result in severe intellectual disability, epilepsy and autism. The primary function of IQSEC2 is to serve as a guanine exchange factor (GEF) controlling the activation of ARF6 which in turn mediates membrane trafficking and synaptic connections between neurons. As IQSEC2 is a large intrinsically disordered protein little is known of the structure of the protein and how this influences its function. Understanding this structure and function relationship is critical for the development of novel therapies to treat IQSEC2 disease. We therefore sought to identify IQSEC2 conformers in unfolded and folded states and analyze how conformers differ when binding to ARF6 and thereby influence GEF catalysis. We simulated the folding process of IQSEC2 by accelerated molecular dynamics (aMD). Following the ensemble method of Gibbs, we proposed that the number of microstates in the ensemble replicating a protein macroscopic system is the total number of MD snapshots sampled on the production MD trajectory. We divided the entire range of reaction coordinate into a series of consecutive, non-overlapping bins. Thermal fluctuations of biomolecules in local equilibrium states are Gaussian in form. To predict the free energy and entropy of different conformational states using statistical thermodynamics, the density of states was estimated taking into account how many MD snapshots constitute each conformational state. IQSEC2 dimers derived from the most stable folded and unfolded conformers of IQSEC2 were generated by protein-protein docking and then used to construct IQSEC2-ARF6 encounter complexes. We suggest that IQSEC2 folding and dimerization are two competing processes that may be used by nature to regulate the process of GDP exchange on ARF6 catalyzed by IQSEC2.
通过统计热力学分析 IQSEC2 蛋白的构象
IQSEC2 基因突变会导致严重的智力障碍、癫痫和自闭症。IQSEC2 的主要功能是作为鸟嘌呤交换因子(GEF),控制 ARF6 的激活,而 ARF6 又反过来介导膜贩运和神经元之间的突触连接。由于 IQSEC2 是一种大型内在无序蛋白,人们对其结构以及结构如何影响其功能知之甚少。了解这种结构与功能的关系对于开发治疗 IQSEC2 疾病的新型疗法至关重要。因此,我们试图识别 IQSEC2 在折叠和未折叠状态下的构象,并分析构象与 ARF6 结合时的差异,从而影响 GEF 催化作用。我们通过加速分子动力学(aMD)模拟了 IQSEC2 的折叠过程。按照吉布斯的集合方法,我们提出,在复制蛋白质宏观系统的集合中,微观状态的数量就是在生成的 MD 轨迹上采样的 MD 快照总数。我们将整个反应坐标范围划分为一系列连续、不重叠的区间。处于局部平衡态的生物大分子的热波动是高斯形式的。为了利用统计热力学预测不同构象状态的自由能和熵,在估算状态密度时考虑了构成每个构象状态的 MD 快照数量。IQSEC2 二聚体来自 IQSEC2 最稳定的折叠和展开构象,通过蛋白质-蛋白质对接生成,然后用于构建 IQSEC2-ARF6 遭遇复合物。我们认为,IQSEC2的折叠和二聚化是两个相互竞争的过程,自然界可能利用这两个过程来调节IQSEC2催化的ARF6上的GDP交换过程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


