Novel duplex TaqMan-based quantitative PCR for rapid and accurate diagnosis of Leishmania (Mundinia) martiniquensis and Leishmania (Mundinia) orientalis, responsible for autochthonous leishmaniasis in Thailand
IF 1.7
Q3 PARASITOLOGY
Current research in parasitology & vector-borne diseases
Pub Date : 2024-01-01
DOI:10.1016/j.crpvbd.2024.100217
引用次数: 0
Abstract
The World Health Organization has recently declared Thailand a leishmaniasis hotspot in Southeast Asia due to the continuous increase in new symptomatic and asymptomatic cases over the years. This emerging parasitic disease is known to be caused by two autochthonous species of Leishmania belonging to the newly described subgenus Mundinia, namely L. martiniquensis and L. orientalis. In Thailand, clinical cases due to L. martiniquensis typically present with visceral leishmaniasis, whereas L. orientalis mainly causes localized cutaneous leishmaniasis. Although Leishmania species confirmation is essential for clinical diagnosis and treatment planning, the availability of highly accurate and rapid diagnostic methods remains limited. In this study, we developed a duplex TaqMan quantitative PCR assay using newly designed species-specific primers and probes based on sequences from the nucleotide and genome databases of Leishmania spp. retrieved from GenBank. The duplex qPCR assay was optimized to specifically amplify the internal transcribed spacer 1 (ITS1) of L. martiniquensis and the heat shock protein 70 (type I) intergenic region (HSP70-I IR) of L. orientalis with high amplification efficiencies. The performance of the optimized duplex qPCR was evaluated by analyzing 46 DNA samples obtained from cultures, and clinical and insect specimens, consistent with the results of the previously validated 18S rRNA-qPCR and ITS1-PCR. The duplex qPCR could detect both species of Leishmania at a limit of detection of one copy per reaction and did not cross-amplify with other pathogen DNA samples. Standard curves of the singleplex and duplex assays showed good linearity with excellent amplification efficiency. Using conventional ITS1-PCR and plasmid sequencing as a reference standard assay, the duplex qPCR showed diagnostic sensitivity and specificity of 100% and positive and negative predictive values of 100% for both Leishmania species with a perfect level of agreement (kappa = 1.0). The novel duplex TaqMan-based qPCR has shown to be a rapid, cost-effective, and highly accurate diagnostic tool for the simultaneous detection and identification of two autochthonous Leishmania spp. in a variety of clinical and entomological samples. This will greatly facilitate early diagnosis, treatment monitoring, and surveillance, especially in leishmaniasis-endemic areas where sequencing-based diagnosis is not routinely available.
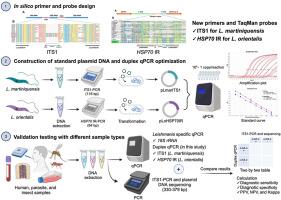
基于 TaqMan 的新型双链定量 PCR,用于快速准确诊断泰国自发性利什曼病的利什曼原虫(Mundinia)martiniquensis 和利什曼原虫(Mundinia)orientalis
世界卫生组织最近宣布泰国为东南亚利什曼病热点地区,原因是多年来新出现的有症状和无症状病例持续增加。据了解,这种新出现的寄生虫病是由属于新描述的 Mundinia 亚属的两种利什曼原虫引起的,即 L. martiniquensis 和 L. orientalis。在泰国,马氏利什曼原虫引起的临床病例通常表现为内脏利什曼病,而东方利什曼原虫则主要引起局部皮肤利什曼病。虽然利什曼原虫的种类确认对于临床诊断和治疗计划至关重要,但高精度和快速诊断方法的可用性仍然有限。在这项研究中,我们根据从 GenBank 检索到的利什曼原虫核苷酸和基因组数据库中的序列,使用新设计的物种特异性引物和探针,开发了一种双联 TaqMan 定量 PCR 检测方法。经优化的双联 qPCR 分析法可特异性扩增马氏利什曼原虫的内部转录间隔区 1(ITS1)和东方利什曼原虫的热休克蛋白 70(I 型)基因间区(HSP70-I IR),且扩增效率高。通过分析从培养物、临床标本和昆虫标本中获得的 46 份 DNA 样本,对优化的双联 qPCR 性能进行了评估,结果与之前验证的 18S rRNA-qPCR 和 ITS1-PCR 结果一致。双联 qPCR 可以检测到两种利什曼原虫,检测限为每个反应一个拷贝,并且不会与其他病原体 DNA 样品发生交叉扩增。单重和双重检测的标准曲线显示出良好的线性关系和极高的扩增效率。以传统的 ITS1-PCR 和质粒测序作为参考标准测定,双联 qPCR 对两种利什曼原虫的诊断灵敏度和特异性均为 100%,阳性和阴性预测值均为 100%,且完全一致(kappa = 1.0)。基于 TaqMan 的新型双联 qPCR 是一种快速、经济、高度准确的诊断工具,可在各种临床和昆虫学样本中同时检测和鉴定两种自生利什曼原虫。这将极大地促进早期诊断、治疗监测和监视,特别是在利什曼病流行地区,因为那里还没有常规的基于测序的诊断方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


