Combined fluorometric analysis of biliverdin and bilirubin by the recombinant protein HUG
IF 1.9
Q2 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
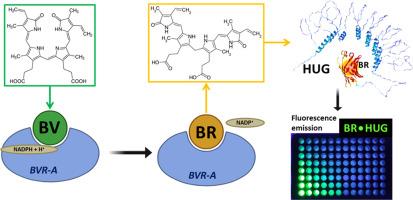
Biliverdin is a secondary metabolite of heme catabolism. It is formed by the reaction catalyzed by heme oxygenase, which converts the heme group contained in proteins such as hemoglobin, myoglobin, cytochromes, and catalase into biliverdin, iron (II) and CO in equimolar amounts, consuming NADPH. Biliverdin is then reduced to bilirubin by biliverdin reductase. Biliverdin and bilirubin form a redox couple and are important for the redox homeostasis of cells. Heme oxygenase-1 is an inducible enzyme that is induced by hypoxic conditions, increased availability of heme or proinflammatory mechanisms such as LPS, UV radiation, etc. In addition, both heme oxygenase-1 and biliverdin reductase play roles other than catalysis by modulating specific metabolic pathways at the transcriptional level. There is a need for affordable assays to analyze these bile pigments in biological and clinical samples. Here we present a method for the combined determination of biliverdin and bilirubin that utilizes the specific binding of bilirubin to the fluorescent recombinant fusion protein HUG and the enzymatic conversion of biliverdin to bilirubin.
- •This method enables the combined measurement of bilirubin and biliverdin in the nM range.
- •The method does not require solvent extraction or protein precipitation of the samples.
利用重组蛋白 HUG 对胆红素和胆红素进行联合荧光分析
胆绿素是血红素分解代谢的次级代谢产物。它由血红素加氧酶催化反应生成,该酶将血红蛋白、肌红蛋白、细胞色素和过氧化氢酶等蛋白质中所含的血红素基转化为等摩尔量的胆绿素、铁(II)和 CO,同时消耗 NADPH。然后,胆红素被胆红素还原酶还原成胆红素。胆红素和胆红素形成氧化还原偶联物,对细胞的氧化还原平衡非常重要。血红素加氧酶-1 是一种诱导型酶,缺氧条件、血红素供应量增加或 LPS、紫外线辐射等促炎机制都会诱导这种酶。此外,血红素加氧酶-1 和胆绿素还原酶除了催化作用外,还在转录水平上调节特定的代谢途径。我们需要经济实惠的检测方法来分析生物和临床样本中的这些胆色素。我们在此介绍一种联合测定胆红素和胆红素的方法,该方法利用胆红素与荧光重组融合蛋白 HUG 的特异性结合以及胆红素向胆红素的酶转化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

MethodsX
Health Professions-Medical Laboratory Technology
CiteScore
3.60
自引率
5.30%
发文量
314
审稿时长
7 weeks
期刊介绍:
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


