Echocardiographic Diagnosis of Hypertrophic Cardiomyopathy by Machine Learning
引用次数: 0
Abstract
Objective
To develop machine learning tools for automated hypertrophic cardiomyopathy (HCM) case recognition from echocardiographic metrics, aiming to identify HCM from standard echocardiographic data with high performance.
Patients and Methods
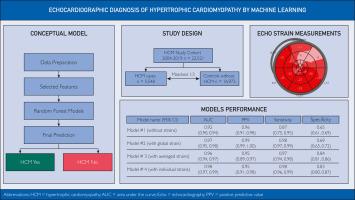
Four different random forest machine learning models were developed using a case-control cohort composed of 5548 patients with HCM and 16,973 controls without HCM, from January 1, 2004, to March 15, 2019. Each patient with HCM was matched to 3 controls by sex, age, and year of echocardiography. Ten-fold crossvalidation was used to train the models to identify HCM. Variables included in the models were demographic characteristics (age, sex, and body surface area) and 16 standard echocardiographic metrics.
Results
The models were differentiated by global, average, individual, or no strain measurements. Area under the receiver operating characteristic curves (area under the curve) ranged from 0.92 to 0.98 for the 4 separate models. Area under the curves of model 2 (using left ventricular global longitudinal strain; 0.97; 95% CI, 0.95-0.98), 3 (using averaged strain; 0.96; 95% CI, 0.94-0.97), and 4 (using 17 individual strains per patient; 0.98; 95% CI, 0.97-0.99) had comparable performance. By comparison, model 1 (no strain data; 0.92; 95% CI, 0.90-0.94) had an inferior area under the curve.
Conclusion
Machine learning tools that analyze echocardiographic metrics identified HCM cases with high performance. Detection of HCM cases improved when strain data was combined with standard echocardiographic metrics.
通过机器学习诊断肥厚型心肌病的超声心动图
目标开发用于从超声心动图指标自动识别肥厚型心肌病(HCM)病例的机器学习工具,旨在从标准超声心动图数据中高效识别 HCM。根据性别、年龄和超声心动图检查年份,每名 HCM 患者与 3 名对照者配对。使用十次交叉验证来训练识别 HCM 的模型。模型中的变量包括人口统计学特征(年龄、性别和体表面积)和 16 项标准超声心动图指标。4 个独立模型的接收者操作特征曲线下面积(曲线下面积)从 0.92 到 0.98 不等。模型 2(使用左心室整体纵向应变;0.97;95% CI,0.95-0.98)、模型 3(使用平均应变;0.96;95% CI,0.94-0.97)和模型 4(使用每位患者 17 个单个应变;0.98;95% CI,0.97-0.99)的曲线下面积表现相当。相比之下,模型 1(无应变数据;0.92;95% CI,0.90-0.94)的曲线下面积较低。当应变数据与标准超声心动图指标相结合时,对 HCM 病例的检测效果更好。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Mayo Clinic Proceedings. Digital health
Medicine and Dentistry (General), Health Informatics, Public Health and Health Policy
自引率
0.00%
发文量
0
审稿时长
47 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


