Anticancer effect of new cyclocoumarol derivatives
European Journal of Medicinal Chemistry Reports
Pub Date : 2024-09-27
DOI:10.1016/j.ejmcr.2024.100220
引用次数: 0
Abstract
Coumarins have demonstrated a broad spectrum of pharmacological activities, including significant anticancer properties. Recently, a series of cyclocoumarol derivatives, a pyranocoumarin known for its anticoagulant and anti-inflammatory effects, have been synthesized and shown to have a selective inhibitory effect on cyclooxygenase-2 (COX-2). In this study, we evaluated the anticancer effects of these cyclocoumarol derivatives for the first time. We tested their antiproliferative effects on several human cancer cell lines, including MDAMB231, A549, MCF7, SF268, HCT116, HeLa, and Jurkat. The MTT assay revealed that the methylated cyclocoumarol derivative, 2-methoxy-2-methyl-(1-(p-tolyl))-3,4-dihydropyrano [3,2-c] chromen-5(2H)-one, 5b exhibited the most potent antiproliferative activity, particularly against MDAMB231 cells. Further investigations demonstrated that 5b induced apoptosis in MDAMB231 cells via the mitochondrial pathway, as indicated by increased Bax and decreased Bcl2 levels, along with caspase-3 activation and PARP cleavage. Additionally, compound 5b significantly inhibited the clonogenic potential of MCF7 cells and reduced the migration capacity of MDAMB231, SF268, and A549 cells. These findings suggest that 5b is a promising lead compound for developing new anticancer agents, with the potential for chemical optimization to enhance its efficacy.
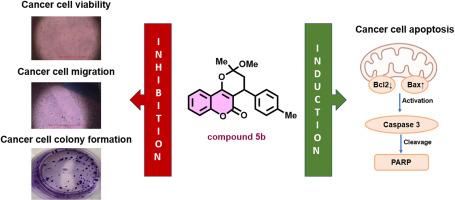
新型环香豆素衍生物的抗癌作用
香豆素具有广泛的药理活性,包括显著的抗癌特性。最近,人们合成了一系列环香豆素衍生物(一种以抗凝血和抗炎作用而闻名的吡喃香豆素),并证明它们对环氧合酶-2(COX-2)具有选择性抑制作用。在本研究中,我们首次评估了这些环香豆素衍生物的抗癌作用。我们测试了它们对几种人类癌细胞株的抗增殖作用,包括 MDAMB231、A549、MCF7、SF268、HCT116、HeLa 和 Jurkat。MTT 试验显示,甲基化的环香豆素衍生物 2-甲氧基-2-甲基-(1-(对甲苯基))-3,4-二氢吡喃并[3,2-c]色烯-5(2H)-酮 5b 表现出最强的抗增殖活性,尤其是对 MDAMB231 细胞。进一步的研究表明,5b 通过线粒体途径诱导 MDAMB231 细胞凋亡,表现为 Bax 水平升高、Bcl2 水平降低、caspase-3 激活和 PARP 断裂。此外,化合物 5b 还能明显抑制 MCF7 细胞的克隆生成潜能,降低 MDAMB231、SF268 和 A549 细胞的迁移能力。这些研究结果表明,5b 是一种很有前景的先导化合物,可用于开发新的抗癌药物,并有可能通过化学优化提高其药效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


