Design, synthesis, and biological evaluation of novel 5,7,4′-trimethoxyflavone sulfonamide-based derivatives as highly potent inhibitors of LRPPRC/STAT3/CDK1
IF 4.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
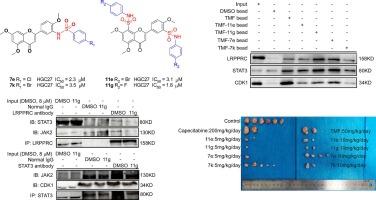
Leucine-rich pentatricopeptide repeat-containing protein (LRPPRC), signal transducer and activator of transcription 3 (STAT3), and cyclin-dependent kinase 1 (CDK1) are promising therapeutic targets for cancer treatment. However, there is a lack of effective inhibitors of LRPPRC, STAT3, and CDK1 in clinic. Our previous study has proved that 5,7,4′-Trimethoxyflavone (TMF) is a novel inhibitor of LRPPRC/STAT3/CDK1. However, the extraction rate of TMF from Tangerine Peel is quite low, and the doses of TMF in cells and mice are rather high. Herein, structural modifications of TMF have led to two series of TMF derivatives including sulfonamide substituted at 3′-position (7a–m) and 3′,8-position (11a–m). Among all compounds, 7e, 7k, 11e, and 11g exhibited as effective, broad-spectrum, and potent anticancer agents in vitro. Moreover, 7e, 7k, 11e, and 11g showed better antitumor effects than TMF and clinical used chemotherapy drug capecitabine in vivo with no obvious toxicity. Mechanism studies showed that 11g could bind to LRPPRC, STAT3, and CDK1 to disassociate the LRPPRC-JAK2-STAT3 and JAK2-STAT3-CDK1 complexes, resulting in suppression of JAK2/STAT3 signaling pathway. These findings suggest that 11g may serve as a leading compound for cancer therapy as a triple-target (LRPPRC, STAT3, and CDK1) inhibitor.
新型 5,7,4′-三甲氧基黄酮磺酰胺基衍生物作为 LRPPRC/STAT3/CDK1 高效抑制剂的设计、合成和生物学评价
富亮氨酸五肽重复序列蛋白(LRPPRC)、转录信号转导和激活因子 3(STAT3)以及细胞周期蛋白依赖性激酶 1(CDK1)是治疗癌症的有望靶点。然而,临床上缺乏有效的 LRPPRC、STAT3 和 CDK1 抑制剂。我们之前的研究证明,5,7,4′-三甲氧基黄酮(TMF)是一种新型的 LRPPRC/STAT3/CDK1 抑制剂。然而,从橘皮中提取 TMF 的提取率很低,而且 TMF 在细胞和小鼠中的剂量相当高。在此,通过对 TMF 进行结构改造,得到了两个系列的 TMF 衍生物,包括 3′ 位(7a-m)和 3′,8-位(11a-m)的磺酰胺取代物。在所有化合物中,7e、7k、11e 和 11g 在体外显示出有效、广谱和强效的抗癌作用。此外,7e、7k、11e 和 11g 在体内的抗肿瘤效果优于 TMF 和临床常用化疗药物卡培他滨,且无明显毒性。机理研究表明,11g 可与 LRPPRC、STAT3 和 CDK1 结合,使 LRPPRC-JAK2-STAT3 和 JAK2-STAT3-CDK1 复合物脱离,从而抑制 JAK2/STAT3 信号通路。这些发现表明,11g 可作为一种三靶点(LRPPRC、STAT3 和 CDK1)抑制剂,成为癌症治疗的主要化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


