The precipitation mechanisms of scheelite from CO2-rich hydrothermal fluids: Insight from thermodynamic modeling
IF 3.1
3区 地球科学
Q1 GEOCHEMISTRY & GEOPHYSICS
引用次数: 0
Abstract
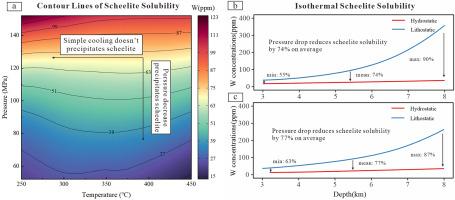
Scheelite and wolframite are two main tungsten-bearing ore minerals in tungsten deposits. Compared to wolframite formed mostly by NaCl–H2O fluids, scheelite in many but not all tungsten deposits is associated with CO2–bearing hydrothermal fluids, but its precipitation mechanisms from these fluids are poorly understood. The replacement of wolframite by scheelite is common among tungsten deposits where these two tungstates coexist, but how CO2, as the pH-buffering agent, affects their replacement remains unclear. To answer these questions, we first tested the pH-buffering effect of CO2 by comparing available experimental pH values of H2O–CO2±NaCl solutions at 200–280 °C and the corresponding thermodynamic modeling results. The mean absolute errors between the calculated and experimental pH values are 0.14 log units in H2O–CO2 solutions and 0.13 log units in H2O–CO2–NaCl solutions, indicating that the calculated acitivities of H+ in CO2-bearing solutions are reliable. Then, the solubilities of scheelite, scheelite-ferberite, and scheelite-hübnerite in NaCl–H2O–CO2 systems were modeled in this study. Our modeling results suggest that scheelite solubility in CO2-rich fluids is highly dependent on fluid pressure but is insensitive to fluid temperature. A decrease in fluid pressure from lithostatic to hydrostatic levels at a depth of 3–8 km causes CO2 escape and pH rise and precipitates over 70 % of tungsten contents in fluids on average. Therefore, CO2 escape is an efficient mechanism for precipitating scheelite from CO2–rich hydrothermal fluids. The independence of scheelite solubility on temperature at high pressures and the slightly retrograde solubility at low pressures allows CO2–rich hydrothermal fluids to carry a great amount of tungsten far away from its sources. This may be one of reasons why some scheelite deposits occur at greater distances (>500 m) from the granite or extend along its strike for several kilometers. Similar to the cases in NaCl–H2O systems, two mechanisms for scheelite replacing wolframite in CO2–rich fluids are identified, a single decrease in fluid pressure or simple cooling plus an increase in the ratios of total Ca contents to total Fe (or Mn) contents in fluids.
富二氧化碳热液中白钨矿的沉淀机制:热力学模型的启示
白钨矿和黑钨矿是钨矿床中两种主要的含钨矿石。与主要由 NaCl-H2O 流体形成的黑钨矿相比,许多(但不是所有)钨矿床中的白钨矿都与含二氧化碳的热液有关,但人们对这些流体中白钨矿的沉淀机制知之甚少。在这两种钨酸盐共存的钨矿床中,黑钨矿被白钨矿取代的现象很常见,但二氧化碳作为pH值缓冲剂如何影响它们的取代仍不清楚。为了回答这些问题,我们首先通过比较 200-280 ℃ 下 H2O-CO2±NaCl 溶液的现有实验 pH 值和相应的热力学建模结果,测试了二氧化碳的 pH 缓冲作用。在 H2O-CO2 溶液和 H2O-CO2-NaCl 溶液中,pH 计算值和实验值之间的平均绝对误差分别为 0.14 和 0.13 个对数单位,这表明在含 CO2 溶液中 H+ 的计算活性是可靠的。随后,本研究建立了白钨矿、白钨矿-铁硼硅石和白钨矿-胡白铁矿在 NaCl-H2O-CO2 体系中的溶解度模型。建模结果表明,白钨矿在富含二氧化碳的流体中的溶解度高度依赖于流体压力,但对流体温度并不敏感。在3-8千米深处,流体压力从岩石静压降低到流体静压水平,会导致二氧化碳逸出和pH值上升,并使流体中平均超过70%的钨含量沉淀下来。因此,二氧化碳逸出是白钨矿从富含二氧化碳的热液中沉淀出来的有效机制。在高压下,白钨矿的溶解度与温度无关,而在低压下,白钨矿的溶解度略有倒退,这使得富含二氧化碳的热液能够将大量的钨带到远离其来源的地方。这可能是一些白钨矿床出现在距离花岗岩较远(500米)或沿花岗岩走向延伸数公里的原因之一。与NaCl-H2O系统中的情况类似,在富含二氧化碳的流体中,白钨矿取代黑钨矿的机制有两种,一种是流体压力的单一降低,另一种是流体中总钙含量与总铁(或锰)含量之比增加的简单冷却。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Geochemistry
地学-地球化学与地球物理
CiteScore
6.10
自引率
8.80%
发文量
272
审稿时长
65 days
期刊介绍:
Applied Geochemistry is an international journal devoted to publication of original research papers, rapid research communications and selected review papers in geochemistry and urban geochemistry which have some practical application to an aspect of human endeavour, such as the preservation of the environment, health, waste disposal and the search for resources. Papers on applications of inorganic, organic and isotope geochemistry and geochemical processes are therefore welcome provided they meet the main criterion. Spatial and temporal monitoring case studies are only of interest to our international readership if they present new ideas of broad application.
Topics covered include: (1) Environmental geochemistry (including natural and anthropogenic aspects, and protection and remediation strategies); (2) Hydrogeochemistry (surface and groundwater); (3) Medical (urban) geochemistry; (4) The search for energy resources (in particular unconventional oil and gas or emerging metal resources); (5) Energy exploitation (in particular geothermal energy and CCS); (6) Upgrading of energy and mineral resources where there is a direct geochemical application; and (7) Waste disposal, including nuclear waste disposal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


