Enhanced Thermodynamic Stability of Delithiated Nano-LiCoO2 by Lanthanum Doping
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
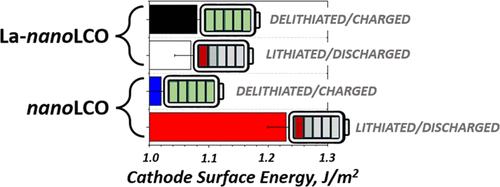
The dynamic environment within lithium-ion batteries induces significant changes in local thermodynamic functions, hampering the accurate prediction of the stability of the cathodes during cycling. While delithiation primarily affects the surface properties of the cathode structure, there is a lack of fundamental understanding concerning the evolution of interfacial energies with varying stoichiometry. Here, we used microcalorimetry to quantify the thermodynamic changes between the stoichiometric and partially delithiated nano-LiCoO2 states for the first time. A mild delithiation from LiCoO2 to Li0.71CoO2 caused a surface energy reduction, negatively affecting the adhesion between adjacent grains by ∼0.4J/m2. The introduction of lanthanum at 1.0 atom % reduced the surface energy of the stoichiometric LiCoO2 while forcing a constant surface energy state during delithiation down to Li0.57CoO2. This reduced the thermodynamic stress between grains during lithium cycling, mitigating degradation mechanisms. The lanthanum-induced surface stabilization also inhibited the coarsening and dissolution of the cathode particles. We used electron microscopy to propose an atomistic mechanism by which the lanthanum doping pins surface dissolution for improved cathode stability.
通过掺杂镧提高二氚化纳米钴酸锂的热力学稳定性
锂离子电池内的动态环境会引起局部热力学函数的显著变化,从而阻碍了对循环过程中阴极稳定性的准确预测。虽然脱硅主要影响阴极结构的表面特性,但人们对不同化学计量的界面能量演变缺乏基本了解。在此,我们首次使用微量热仪量化了化学计量态和部分脱锂纳米钴酸锂态之间的热力学变化。从LiCoO2到Li0.71CoO2的轻度脱硫导致表面能降低,对相邻晶粒间的粘附力产生了0.4J/m2的负面影响。引入 1.0 原子%的镧降低了化学计量钴酸锂的表面能,同时迫使脱硫过程中的表面能状态保持不变,降至 Li0.57CoO2。这降低了锂循环过程中晶粒间的热力学应力,减轻了降解机制。镧诱导的表面稳定化还抑制了正极颗粒的粗化和溶解。我们利用电子显微镜提出了镧掺杂抑制表面溶解以提高阴极稳定性的原子机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


