Oxygen Storage Capacity of Y0.8Ca0.2BaCo4 – xMxO7 + δ (M = Fe, Ga, Al; 0 < x < 1) Solid Solutions during Thermal Cycling in Air
IF 0.9
4区 材料科学
Q4 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
We have studied the behavior of Y1 – yCayBaCo4 – xMxO7 + δ solid solutions in cyclic oxygen absorption/release processes in air at temperatures in the range 350–580°C. Y0.8Ca0.2BaCoO7 + δ has been found to absorb the largest amount of oxygen: 0.52 wt % (325 μmol O/g). The incorporation of calcium and iron into the structure of the YBaCo4O7 + δ cobaltite has been shown to shift the oxygen exchange process to higher temperatures and increase the oxygen storage capacity of the material.
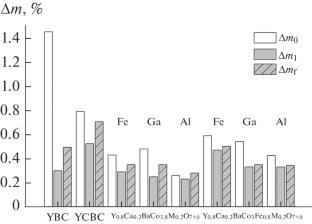
Y0.8Ca0.2BaCo4 - xMxO7 + δ(M = Fe、Ga、Al;0 < x < 1)固溶体在空气中热循环时的储氧能力
我们研究了 Y1 - yCayBaCo4 - xMxO7 + δ 固溶体在温度为 350-580°C 的空气中循环吸收/释放氧气的过程。研究发现,Y0.8Ca0.2BaCoO7 + δ 的吸氧量最大:0.52 wt %(325 μmol O/g)。在 YBaCo4O7 + δ 钴酸盐结构中加入钙和铁,可将氧交换过程转移到更高的温度,并提高材料的储氧能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Materials
工程技术-材料科学:综合
CiteScore
1.40
自引率
25.00%
发文量
80
审稿时长
3-6 weeks
期刊介绍:
Inorganic Materials is a journal that publishes reviews and original articles devoted to chemistry, physics, and applications of various inorganic materials including high-purity substances and materials. The journal discusses phase equilibria, including P–T–X diagrams, and the fundamentals of inorganic materials science, which determines preparatory conditions for compounds of various compositions with specified deviations from stoichiometry. Inorganic Materials is a multidisciplinary journal covering all classes of inorganic materials. The journal welcomes manuscripts from all countries in the English or Russian language.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


