Aluminum Dihalide Complexes Based on the 1,2-bis[(2,6-dibenzhydryl-4-methylphenyl)imino]Acenaphthene Ligand
IF 1.2
4区 化学
Q4 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
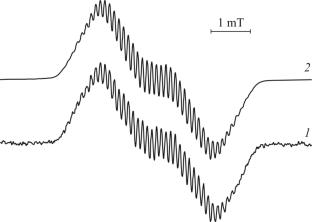
Reduction of ArBIG-bian (ArBIG-bian = 1,2-bis[(2,6-dibenzhydryl-4-methylphenyl)imino]acenaphthene) by an excess of metal aluminum in the presence of AlCl3, AlBr3 or AlI3 in toluene leads to the formation of dihalide anion-radical complexes [(ArBIG-bian)–AlX2] (X = Cl (1), Br (2), I (3)). The newly obtained compounds 1, 2, 3 are isolated in the crystal state and characterized by IR and EPR spectroscopies and by elemental analysis. Molecular structures of the complexes are determined by XRD.
基于 1,2-双[(2,6-二苯甲基-4-甲基苯基)亚氨基]苊配体的二卤化铝配合物
在 AlCl3、AlBr3 或 AlI3 的存在下,过量的金属铝在甲苯中还原 ArBIG-bian(ArBIG-bian = 1,2-双[(2,6-二苯甲酰基-4-甲基苯基)亚氨基]苊),形成二卤化阴离子-自由基配合物 [(ArBIG-bian)-AlX2] (X = Cl (1)、Br (2)、I (3))。新得到的化合物 1、2、3 分离出晶体,并通过红外光谱、EPR 光谱和元素分析进行了表征。复合物的分子结构由 XRD 确定。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Structural Chemistry
化学-无机化学与核化学
CiteScore
1.60
自引率
12.50%
发文量
142
审稿时长
8.3 months
期刊介绍:
Journal is an interdisciplinary publication covering all aspects of structural chemistry, including the theory of molecular structure and chemical bond; the use of physical methods to study the electronic and spatial structure of chemical species; structural features of liquids, solutions, surfaces, supramolecular systems, nano- and solid materials; and the crystal structure of solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


