Binding characteristics of the major kratom alkaloid, mitragynine, towards serum albumin: Spectroscopic, calorimetric, microscopic, and computational investigations
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Mitragynine (MTG) is a prominent indole alkaloid that is present abundantly in Mitragyna speciosa, commonly referred to as kratom. MTG has garnered significant attention due to its selective agonistic characteristics towards opioid receptors and related analgesic effects. In the circulatory system, the in vivo efficacy of MTG is dictated by its interaction with plasma proteins, primarily human serum albumin (HSA). In the present study, we utilized a broad methodology that included spectroscopic, calorimetric, microscopic, and in silico approaches to characterize the interaction between MTG and HSA. Alterations in the UV absorption spectrum of HSA by the presence of MTG demonstrated a ground-state complexation between the protein and the ligand. The Ka values obtained for the MTG–HSA interaction were in the range 103–104 M−1 based on analysis of fluorescence and ITC data, respectively, indicating an intermediate binding affinity. The binding reaction was thermodynamically favorable as revealed by ΔH, ΔS, and ΔG values of −16.42 kJ mol−1, 39.97 J mol−1 K−1, and −28.34 kJ mol−1, respectively. Furthermore, CD spectroscopy results suggested MTG binding induced minimal effects on the structural integrity of HSA, supported by computational methods. Changes in the dimensions of HSA particles due to aggregation, as observed using atomic force microscopy in the presence of MTG. Competitive drug displacement results seemingly suggested site III of HSA located at subdomain IB as the preferred binding site of MTG, but were in inconclusive. However, docking results showed the clear preference of MTG to bind to site III, facilitated by hydrophobic (alkyl and pi-alkyl) and van der Waals forces, together with carbon hydrogen bonds. Additionally, the MTG–HSA complexation was demonstrated to be stable based on molecular dynamics analysis. The outcomes of this study shed light on the therapeutic potential of MTG and can help in the design of more effective derivatives of the compound.
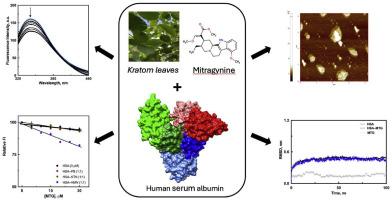
主要桔梗生物碱--丝氨酸与血清白蛋白的结合特性:光谱、量热、显微和计算研究。
Mitragynine (MTG) 是一种突出的吲哚生物碱,大量存在于 Mitragyna speciosa(俗称桔梗)中。MTG 因其对阿片受体的选择性激动特性和相关镇痛效果而备受关注。在循环系统中,MTG 的体内药效取决于它与血浆蛋白(主要是人血清白蛋白(HSA))的相互作用。在本研究中,我们采用了一种广泛的方法,包括光谱、量热、显微和硅学方法,来描述 MTG 与 HSA 之间相互作用的特征。MTG 的存在改变了 HSA 的紫外吸收光谱,这表明蛋白质与配体之间存在基态复合物。根据对荧光数据和 ITC 数据的分析,MTG 与 HSA 相互作用的 Ka 值分别在 103-104 M-1 之间,表明这是一种中等程度的结合亲和力。ΔH、ΔS 和 ΔG 值分别为 -16.42 kJ mol-1、39.97 J mol-1 K-1 和 -28.34 kJ mol-1,表明结合反应在热力学上是有利的。此外,CD 光谱结果表明,MTG 结合对 HSA 结构完整性的影响微乎其微,这也得到了计算方法的支持。使用原子力显微镜观察到,在 MTG 存在的情况下,HSA 颗粒的尺寸会因聚集而发生变化。药物竞争性置换结果表明,位于亚域 IB 的 HSA 第 III 位点似乎是 MTG 的首选结合位点,但并不确定。然而,对接结果表明,在疏水力(烷基和偏烷基)和范德华力以及碳氢键的作用下,MTG 显然更倾向于与位点 III 结合。此外,分子动力学分析表明 MTG-HSA 复合物是稳定的。这项研究成果揭示了 MTG 的治疗潜力,有助于设计出更有效的化合物衍生物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


