GLTSCR1 deficiency promotes colorectal cancer development through regulating non-homologous end joining
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Non-homologous end joining (NHEJ), as one major pathway of DNA double-strand break (DSB) repair, could cause genomic instability, which plays pivotal roles in cancer development. While, chromatin remodeling complexes dictate the selection and orchestration of DSB repair pathways by regulating chromatin dynamics. However, the crosstalk between NHEJ and chromatin remodeling in cancer progress remains unclear. In this study, deficiency of GLTSCR1 causes resistance to DNA damage in colorectal cancer (CRC) cells by promoting NHEJ repair efficiency. Mechanistically, GLTSCR1 interacts with BRD9 to engage in the assembly of the non-canonical BAF complex (GBAF). However, GLTSCR1 deficiency disrupts GBAF and triggers the ubiquitination degradation of BRD9. Furthermore, GLTSCR1 deficiency causes aberrant opening in the promoter region of NHEJ repair-associated genes, which promotes CRC development. While, GLTSCR1 and its binding partner BRD9 are not directly involved in assembling NHEJ repair machinery; instead, they regulate the DNA accessibility of NHEJ repair-associated genes. Collectively, our findings confirm GLTSCR1 deficiency as a critical regulatory event of the NHEJ pathway in CRC development, which might require different therapeutic strategy for GLTSCR1 wild-type and mutant CRC.
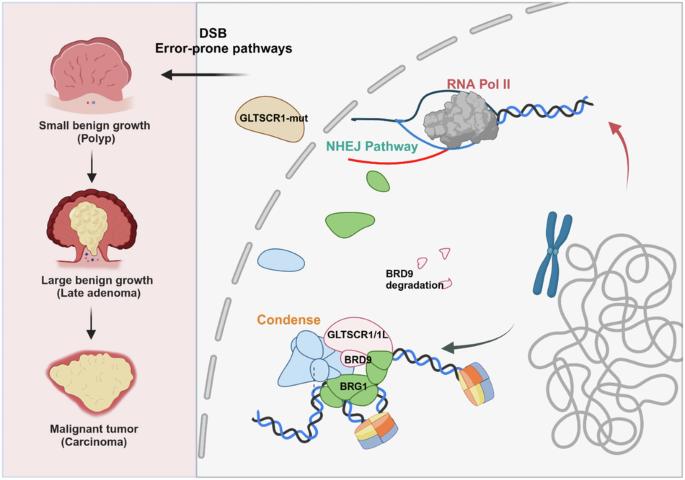
GLTSCR1 缺乏会通过调节非同源末端连接促进结直肠癌的发展。
非同源末端连接(NHEJ)是DNA双链断裂(DSB)修复的一个主要途径,它可能导致基因组不稳定,在癌症的发展中起着关键作用。染色质重塑复合物通过调控染色质动态,决定着DSB修复途径的选择和协调。然而,NHEJ和染色质重塑在癌症进展中的相互影响仍不清楚。在这项研究中,GLTSCR1的缺乏会通过促进NHEJ修复效率而导致结直肠癌(CRC)细胞对DNA损伤的抵抗。从机理上讲,GLTSCR1与BRD9相互作用,参与非典型BAF复合物(GBAF)的组装。然而,缺乏 GLTSCR1 会破坏 GBAF 并引发 BRD9 的泛素化降解。此外,GLTSCR1 缺乏还会导致 NHEJ 修复相关基因启动子区域异常开放,从而促进 CRC 的发展。GLTSCR1及其结合伙伴BRD9并不直接参与组装NHEJ修复机制,而是调节NHEJ修复相关基因的DNA可及性。总之,我们的研究结果证实了 GLTSCR1 缺乏是 NHEJ 通路在 CRC 发展过程中的一个关键调控事件,这可能需要针对 GLTSCR1 野生型和突变型 CRC 采取不同的治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


