SGLT1 inhibition alleviates radiation-induced intestinal damage through promoting mitochondrial homeostasis
IF 7.1
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Radiation-induced intestinal injury (RIII) constitutes a challenge in radiotherapy. Ionizing radiation (IR) induces DNA and mitochondrial damage by increasing reactive oxygen species (ROS). Sodium–glucose cotransporter 1 (SGLT1) is abundant in the gastrointestinal tract and the protective effects of inhibited SGLT1 in kidney and cardiovascular disease have been widely reported. However, the function of SGLT1 in RIII remains unclear. Herein, we reported that IR induced intestinal epithelial cell damage along with upregulation of SGLT1 in vivo and in vitro, which was alleviated by inhibition of SGLT1. Specifically, maintaining intestinal cell homeostasis was detected through cellular proliferation, apoptosis, and DNA damage assays, promoting epithelial regeneration and lifespan extension. Considering the importance of mitochondrial function in cell fate, we next confirmed that SGLT inhibition maintains mitochondrial homeostasis through enhanced mitophagy in intestinal epithelial cells. Finally, based on the bioinformatics analysis and cell validation, we demonstrated that inhibition of SGLT1 suppresses the PI3K/AKT/mTOR pathway to enhance mitophagy activation post-irradiation. In addition, we preliminarily demonstrate that SGLT inhibitors do not affect the radiosensitivity of tumors. Hence, our findings suggest that inhibition of SGLT is a promising therapeutic strategy to protect against RIII. To the best of our knowledge, this is the first report on the potential effect of SGLT1 inhibition in RIII.
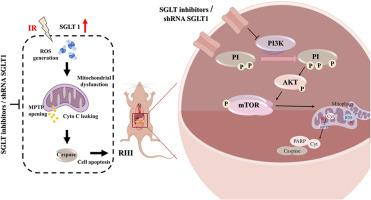
抑制 SGLT1 可通过促进线粒体平衡减轻辐射引起的肠道损伤。
辐射诱导的肠道损伤(RIII)是放疗中的一项挑战。电离辐射(IR)通过增加活性氧(ROS)诱导 DNA 和线粒体损伤。钠-葡萄糖共转运体 1(SGLT1)在胃肠道中含量丰富,抑制 SGLT1 对肾脏和心血管疾病的保护作用已被广泛报道。然而,SGLT1 在 RIII 中的功能仍不清楚。在此,我们报告了红外诱导肠上皮细胞损伤以及体内和体外 SGLT1 的上调,抑制 SGLT1 可减轻损伤。具体来说,通过细胞增殖、凋亡和DNA损伤试验检测到了维持肠细胞稳态的作用,促进了上皮细胞再生和寿命延长。考虑到线粒体功能在细胞命运中的重要性,我们接下来证实了 SGLT 抑制通过增强肠上皮细胞的有丝分裂来维持线粒体的稳态。最后,基于生物信息学分析和细胞验证,我们证明抑制 SGLT1 可抑制 PI3K/AKT/mTOR 通路,从而增强辐照后的有丝分裂活化。此外,我们初步证明 SGLT 抑制剂不会影响肿瘤的放射敏感性。因此,我们的研究结果表明,抑制 SGLT 是一种很有前景的抗 RIII 治疗策略。据我们所知,这是首次报道 SGLT1 抑制剂对 RIII 的潜在影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


