Base-Promoted Chemodivergent Construction of 2H-Chromen-2-one and Chromeno[2,3-c]pyrrole Scaffolds from para-Quinone Methides and α-Alkylidene Succinimides.
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein, we evolve a base-promoted synthesis of 2H-chromen-2-one and chromeno[2,3-c]pyrrole scaffolds via (4 + 2) annulation of α-alkylidene succinimides with 2-hydroxyphenyl-substituted para-quinone methides (p-QMs). Extremely selective and switchable cyclizations were obtained by modifying the base. This metal-free protocol is highlighted by its mild reaction conditions and broad substrate scope, and the viability of the existing protocol was additionally illustrated by gram-scale synthesis and further modification. Several control experiments were performed to understand the reaction mechanism.
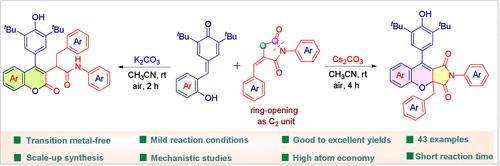
由对位醌甲酰胺和α-亚烷基琥珀酰亚胺在碱促进下构建 2H-色烯-2-酮和色烯并[2,3-c]吡咯支架的化学发散作用。
在此,我们通过α-亚烷基琥珀酰亚胺与 2-羟基苯基取代的对醌甲酰胺(p-QMs)的(4 + 2)环化反应,发展了一种碱促进的 2H-色烯-2-酮和色烯并[2,3-c]吡咯支架的合成方法。通过修改碱基,获得了极具选择性和可切换的环化反应。这种无金属方案因其温和的反应条件和广泛的底物范围而备受瞩目,此外,现有方案的可行性还通过克级规模的合成和进一步修改得到了证实。为了了解反应机理,还进行了一些对照实验。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


