Reduction of myeloid-derived suppressor cells in prostate cancer murine models and patients following white button mushroom treatment
Abstract
Background
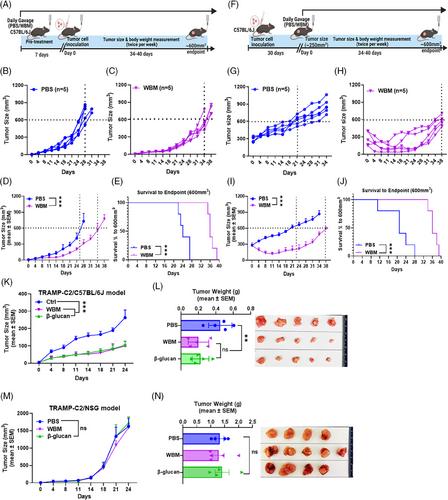
In a previously reported Phase I trial, we observed therapy-associated declines in circulating myeloid-derived suppressor cells (MDSCs) with the administration of white button mushroom (WBM) tablets in prostate cancer (PCa) patients. These observations led us to hypothesise that WBM could mitigate PCa progression by suppressing MDSCs.
Methods
We performed bidirectional translational research to examine the immunomodulatory effects of WBM consumption in both syngeneic murine PCa models and patients with PCa participating in an ongoing randomised Phase II trial (NCT04519879).
Results
In murine models, WBM treatment significantly suppressed tumour growth with a reduction in both the number and function of MDSCs, which in turn promoted antitumour immune responses mediated by T cells and natural killer (NK) cells. In patients, after consumption of WBM tablets for 3 months, we observed a decline in circulating polymorphonuclear MDSCs (PMN-MDSCs), along with an increase in cytotoxic CD8+ T and NK cells. Furthermore, single immune cell profiling of peripheral blood from WBM-treated patients showed suppressed STAT3/IRF1 and TGFβ signalling in circulating PMN-MDSCs. Subclusters of PMN-MDSCs presented transcriptional profiles associated with responsiveness to fungi, neutrophil chemotaxis, leukocyte aggregation, and regulation of inflammatory response. Finally, in mouse models of PCa, we found that WBM consumption enhanced the anticancer activity of anti-PD-1 antibodies, indicating that WBM may be used as an adjuvant therapy with immune checkpoint inhibitors.
Conclusion
Our results from PCa murine models and patients provide mechanistic insights into the immunomodulatory effects of WBM and provide a scientific foundation for WBM as a nutraceutical intervention to delay or prevent PCa progression.
Highlights
- White button mushroom (WBM) treatment resulted in a reduction in pro-tumoural MDSCs, notably polymorphonuclear MDSCs (PMN-MDSCs), along with activation of anti-tumoural T and NK cells.
- Human single immune cell gene expression profiling shed light on the molecular alterations induced by WBM, specifically on PMN-MDSCs.
- A proof-of-concept study combining WBM with PD-1 blockade in murine models revealed an additive effect on tumour regression and survival outcomes, highlighting the clinical relevance of WBM in cancer management.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


