AIE-based ruthenium complexes as photosensitizers for specifically photo-inactivate gram-positive bacteria
IF 3.8
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The emergence of multidrug-resistant bacterial have caused severe burden for public health. Particularly, Staphylococcus aureus as one of ESKAPE pathogens have induced various infectious diseases and resulted in increasing deaths. Developing new antibacterial agents is still urgent and challenging. Fortunately, in this study, based on aggregation-induced emission (AIE) ruthenium complexes were designed and synthesized, which realized the high efficiency of reactive oxygen species generation and remarkably killed S. aureus unlike conventional antibiotics action. Significantly, owing to good singlet oxygen production ability, Ru1 at only 4 μg/mL of concentration displayed good antibacterial photodynamic therapy effect upon white light irradiation and could deplete essential coenzyme NADH to disrupt intracellular redox balance. Also, the electrostatic interaction between Ru1 and bacteria enhanced the possibility of antibacterial. Under light irradiation, Ru1 could efficiently inhibit the biofilm growth and avoid the development of drug-resistant. Furthermore, Ru1 possessed excellent biocompatibility and displayed remarkable therapy effect in treating mice-wound infections in vivo. These findings indicated that AIE-based ruthenium complexes as new antibacterial agent had great potential in photodynamic therapy of bacteria and addressing the drug-resistance crisis.
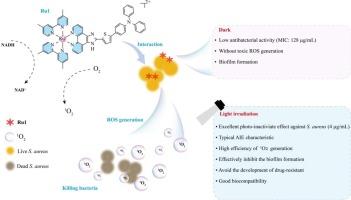
以 AIE 为基础的钌复合物作为光敏剂,专门用于光灭活革兰氏阳性细菌。
耐多药细菌的出现给公共卫生造成了严重负担。特别是金黄色葡萄球菌作为 ESKAPE 病原体之一,诱发了各种传染病,导致越来越多的人死亡。开发新的抗菌剂仍然是一项紧迫而又具有挑战性的任务。幸运的是,本研究设计并合成了基于聚集诱导发射(AIE)的钌复合物,它实现了活性氧的高效生成,与传统抗生素的作用不同,能显著杀灭金黄色葡萄球菌。值得注意的是,由于具有良好的单线态氧生成能力,在白光照射下,浓度仅为 4 μg/mL 的 Ru1 就能显示出良好的光动力抗菌治疗效果,并能消耗细胞内必需的辅酶 NADH,从而破坏细胞内的氧化还原平衡。此外,Ru1 与细菌之间的静电作用也增强了抗菌的可能性。在光照射下,Ru1 能有效抑制生物膜的生长,避免耐药性的产生。此外,Ru1 还具有良好的生物相容性,在治疗体内小鼠伤口感染方面具有显著疗效。这些研究结果表明,基于 AIE 的钌复合物作为新型抗菌剂,在光动力治疗细菌和解决耐药性危机方面具有巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


