Pyrrolo[2,3-d]pyrimidines as potential kinase inhibitors in cancer drug discovery: A critical review
IF 4.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
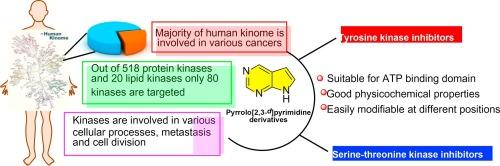
Pyrrolo[2,3-d]pyrimidine-based kinase inhibitors have emerged as an important class of targeted therapeutics to combat various types of cancer. The distinctive structural feature of pyrrolopyrimidine ring system offers an adaptable platform for designing potent inhibitors of various kinases, crucial in regulating cellular processes. The deazapurine framework inherent to pyrrolopyrimidines bears a conspicuous resemblance to adenine, the natural ligand ATP. The structural mimicry enhances their appeal as potent inhibitors of key kinases. This review reconnoitres the intricate process of designing and developing pyrrolopyrimidine based derivatives, accentuating their structural diversity and the strategic modifications employed to enhance selectivity, potency, and pharmacokinetic properties. The discussion delves into medicinal chemistry strategies, highlighting successful examples that have been progressed to clinical evaluation. Furthermore, the review highlights the promise of pyrrolopyrimidine scaffolds in revolutionizing targeted cancer therapy and provides a pioneering perspective on future directions.
作为潜在激酶抑制剂的吡咯并[2,3-d]嘧啶类抗癌药物发现:重要综述。
基于吡咯并[2,3-d]嘧啶的激酶抑制剂已成为抗击各类癌症的一类重要靶向治疗药物。吡咯嘧啶环系统独特的结构特征为设计对调节细胞过程至关重要的各种激酶的强效抑制剂提供了一个适应性很强的平台。吡咯并嘧啶固有的脱氮嘌呤框架与天然配体 ATP 的腺嘌呤十分相似。这种结构拟态增强了它们作为关键激酶强效抑制剂的吸引力。本综述回顾了设计和开发吡咯并嘧啶类衍生物的复杂过程,强调了其结构的多样性以及为提高选择性、药效和药代动力学特性而采用的策略性修饰。讨论深入探讨了药物化学策略,重点介绍了已进入临床评估的成功案例。此外,该综述还强调了吡咯并嘧啶支架在革新癌症靶向治疗方面的前景,并对未来的发展方向提供了开拓性的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


