VEGFB promotes adipose tissue thermogenesis by inhibiting norepinephrine clearance in macrophages
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2024-10-06
DOI:10.1016/j.bbadis.2024.167536
引用次数: 0
Abstract
Adipokines play key roles in adaptive thermogenesis of beige adipocytes, though its detailed regulatory mechanisms are not fully understood. In the present study, we identify a critical function of vascular endothelial growth factor B (VEGFB)/vascular endothelial growth factor receptor 1 (VEGFR1) signaling in improving thermogenesis in white adipose tissue (WAT). In mouse subcutaneous WAT (scWAT), thermogenesis activation leads to the up-regulation of VEGFB in adipocytes and its receptor VEGFR1 in macrophages. Ablation of adipocyte VEGFB results in deficiency in murine WAT browning. Meanwhile, supplementation of VEGFB promotes WAT thermogenesis, but this effect is blocked by knockout of macrophage VEGFR1. Mechanistic studies show that the VEGFB-activated VEGFR1 inhibits p38 MAPK signaling through its dissociation with receptor for activated C kinase 1, thereby preventing norepinephrine transporter (solute carrier family 6 member 2) and norepinephrine-degrative monoamine oxidase a mediated norepinephrine clearance in macrophages. Our findings demonstrate that VEGFB/VEGFR1 circuit contributes to the WAT thermogenesis.
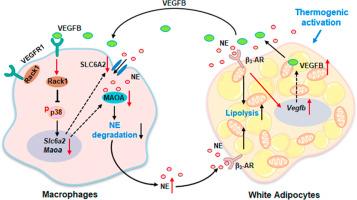
血管内皮生长因子B通过抑制巨噬细胞中去甲肾上腺素的清除来促进脂肪组织产热。
脂肪因子在米色脂肪细胞的适应性产热过程中发挥着关键作用,但其详细的调控机制尚不完全清楚。在本研究中,我们发现了血管内皮生长因子 B(VEGFB)/血管内皮生长因子受体 1(VEGFR1)信号在改善白色脂肪组织(WAT)产热过程中的关键功能。在小鼠皮下脂肪组织(scWAT)中,产热激活会导致脂肪细胞中的血管内皮生长因子B和巨噬细胞中的血管内皮生长因子受体VEGFR1上调。消融脂肪细胞中的 VEGFB 会导致小鼠 WAT 褐化不足。同时,补充 VEGFB 可促进脂肪细胞的产热,但巨噬细胞 VEGFR1 的基因敲除会阻断这种效应。机理研究表明,VEGFB激活的VEGFR1通过与活化C激酶1受体分离抑制p38 MAPK信号转导,从而阻止巨噬细胞中去甲肾上腺素转运体(溶质运载家族6成员2)和去甲肾上腺素降解单胺氧化酶a介导的去甲肾上腺素清除。我们的研究结果表明,血管内皮生长因子B/血管内皮生长因子受体1回路有助于脂肪热生成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


