Bulk integrated single-cell-spatial transcriptomics reveals the impact of preoperative chemotherapy on cancer-associated fibroblasts and tumor cells in colorectal cancer, and construction of related predictive models using machine learning
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2024-10-05
DOI:10.1016/j.bbadis.2024.167535
引用次数: 0
Abstract
Background
Preoperative chemotherapy (PC) is an important component of Colorectal cancer (CRC) treatment, but its effects on the biological functions of fibroblasts and epithelial cells in CRC are unclear.
Methods
This study utilized bulk, single-cell, and spatial transcriptomic sequencing data from 22 independent cohorts of CRC. Through bioinformatics analysis and in vitro experiments, the research investigated the impact of PC on fibroblast and epithelial cells in CRC. Subpopulations associated with PC and CRC prognosis were identified, and a predictive model was constructed using machine learning.
Results
PC significantly attenuated the pathways related to tumor progression in fibroblasts and epithelial cells. NOTCH3 + Fibroblast (NOTCH3 + Fib), TNNT1 + Epithelial (TNNT1 + Epi), and HSPA1A + Epithelial (HSPA1A + Epi) subpopulations were identified in the adjacent spatial region and were associated with poor prognosis in CRC. PC effectively diminished the presence of these subpopulations, concurrently inhibiting pathway activity and intercellular crosstalk. A risk signature model, named the Preoperative Chemotherapy Risk Signature Model (PCRSM), was constructed using machine learning. PCRSM emerged as an independent prognostic indicator for CRC, impacting both overall survival (OS) and recurrence-free survival (RFS), surpassing the performance of 89 previously published CRC risk signatures. Additionally, patients with a high PCRSM risk score showed sensitivity to fluorouracil-based adjuvant chemotherapy (FOLFOX) but resistance to single chemotherapy drugs (such as Bevacizumab and Oxaliplatin). Furthermore, this study predicted that patients with high PCRSM were resistant to anti-PD1therapy.
Conclusion
In conclusion, this study identified three cell subpopulations (NOTCH3 + Fib, TNNT1 + Epi, and HSPA1A + Epi) associated with PC, which can be targeted to improve the prognosis of CRC patients. The PCRSM model shows promise in enhancing the survival and treatment of CRC patients.
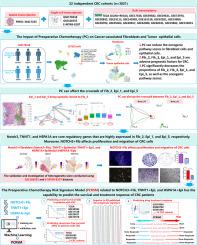
批量整合的单细胞空间转录组学揭示了结直肠癌术前化疗对癌症相关成纤维细胞和肿瘤细胞的影响,并利用机器学习构建了相关预测模型。
背景:术前化疗(PC)是结直肠癌(CRC)治疗的重要组成部分,但其对 CRC 中成纤维细胞和上皮细胞生物功能的影响尚不清楚:本研究利用了来自 22 个独立队列的 CRC 的大量、单细胞和空间转录组测序数据。通过生物信息学分析和体外实验,该研究调查了 PC 对 CRC 中成纤维细胞和上皮细胞的影响。研究发现了与PC和CRC预后相关的亚群,并利用机器学习构建了一个预测模型:结果:PC能明显减弱成纤维细胞和上皮细胞中与肿瘤进展相关的通路。在邻近空间区域发现了NOTCH3 +成纤维细胞(NOTCH3 + Fib)、TNNT1 +上皮细胞(TNNT1 + Epi)和HSPA1A +上皮细胞(HSPA1A + Epi)亚群,它们与CRC的不良预后有关。PC 能有效减少这些亚群的存在,同时抑制通路活性和细胞间串扰。研究人员利用机器学习构建了一个风险特征模型,命名为术前化疗风险特征模型(PCRSM)。PCRSM成为独立的CRC预后指标,对总生存期(OS)和无复发生存期(RFS)都有影响,超过了之前发表的89个CRC风险特征模型。此外,PCRSM风险评分高的患者对以氟尿嘧啶为基础的辅助化疗(FOLFOX)敏感,但对单一化疗药物(如贝伐单抗和奥沙利铂)耐药。此外,本研究还预测 PCRSM 分值高的患者对抗 PD1 治疗具有耐药性:总之,这项研究发现了与 PC 相关的三种细胞亚群(NOTCH3 + Fib、TNNT1 + Epi 和 HSPA1A + Epi),可以针对这些亚群改善 CRC 患者的预后。PCRSM 模型有望提高 CRC 患者的生存率和治疗效果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


