Maternal heart exhibits metabolic and redox adaptations post-uncomplicated pregnancy
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2024-10-06
DOI:10.1016/j.bbadis.2024.167539
引用次数: 0
Abstract
Pregnancy may be a challenging period for the maternal systems and has been regarded as a stress test, as imperceptible/mild dysfunctions eventually present may be exacerbated during this period. The cardiovascular system is no exception, and several morphological and functional adaptations accompanying pregnancy have been described. However, long-term pregnancy-induced cardiac molecular alterations remain highly unexplored. The postpartum is marked by reverse remodeling of the pregnancy-induced cardiovascular adaptations, representing a possible critical period for assessing future maternal cardiovascular health. The current study explored the molecular and metabolic alterations in the cardiac tissue eight weeks after a physiological uncomplicated pregnancy. Female Sprague-Dawley rats were fed a chow diet through pregnancy, lactation, and weaning and compared to their non-pregnant counterparts. Eight weeks postpartum, increased levels of the phosphorylated form of AMPKα (Thr172) and its ratio to total AMPKα indicated possible alterations in cardiac metabolic flexibility, accompanied by increased Pparα and Hif1α transcripts levels. Additionally, postpartum hearts exhibited higher mitochondrial ATP and NADH levels without major changes in mitochondrial respiratory function. Elevated Nrf2 levels in the cardiac tissue suggested potential implications for cardiac redox balance, further supported by increased levels or activity of proteins directly regulated by Nrf2. The findings herein reported suggest that at eight weeks postpartum, molecular alterations induced by pregnancy, especially regarding redox balance, are still observed in the mothers' heart. These alterations present at late postpartum may open new avenues to understand the different risk for cardiovascular complications development after normal pregnancies.
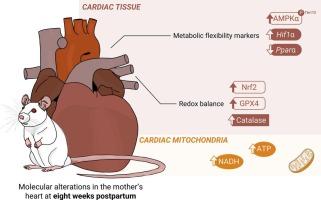
Biochimica et biophysica acta:非并发妊娠后母体心脏出现新陈代谢和氧化还原适应性疾病的分子基础。
妊娠期对母体系统来说是一个充满挑战的时期,被认为是一种压力测试,因为在此期间,最终存在的不易察觉/轻微的功能障碍可能会加剧。心血管系统也不例外,已经描述了伴随妊娠出现的几种形态和功能适应性变化。然而,长期妊娠诱导的心脏分子改变仍是一个尚未探索的领域。产后妊娠诱导的心血管适应性会发生反向重塑,这可能是评估孕产妇未来心血管健康的关键时期。本研究探讨了生理性无并发症妊娠八周后心脏组织的分子和代谢变化。雌性 Sprague-Dawley 大鼠在整个孕期、哺乳期和断奶期都以饲料喂养,并与未怀孕的大鼠进行比较。产后八周,AMPKα的磷酸化形式(Thr172)水平及其与总AMPKα的比率升高,表明心脏代谢灵活性可能发生了改变,同时Pparα和Hif1α转录物水平升高。此外,产后心脏的线粒体 ATP 和 NADH 水平较高,但线粒体呼吸功能没有发生重大变化。心脏组织中 Nrf2 水平的升高表明了对心脏氧化还原平衡的潜在影响,而 Nrf2 直接调控的蛋白质水平或活性的升高也进一步证实了这一点。本文报告的研究结果表明,在产后八周,仍可在母亲的心脏中观察到妊娠引起的分子变化,尤其是氧化还原平衡方面的变化。产后晚期出现的这些变化可能为了解正常妊娠后心血管并发症的不同风险开辟了新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


