Unraveling the mechanisms of RECQL4-mediated cervical cancer progression through the PI3K/AKT pathway
IF 5
2区 医学
Q2 Medicine
引用次数: 0
Abstract
Background
RECQL4 is a member of the DNA helicase family and is critical for DNA replication, DNA damage repair, and tumor progression. However, its specific role in cervical cancer remains uncertain.
Methods
In this study, we aimed to investigate the impact of RECQL4 on cervical cancer prognosis using clinical specimens from The Cancer Genome Atlas. We evaluated the malignant effects of RECQL4 through various experimental assays including cell Cell Counting Kit-8, EdU, colony formation, cell cycle analysis, cell apoptosis, scratch, and Transwell assays. We explored the mechanisms of RECQL4-regulated malignancy using analyses of bioinformatics, RNA sequencing data, polymerase chain reaction (PCR), western blotting, and cell immunofluorescence experiments. Furthermore, we validated the effects of RECQL4 knockdown on tumor growth using subcutaneous tumor models in nude mice.
Results
RECQL4 was upregulated in cervical cancer and correlated with prognosis, demonstrating a positive relationship with tumor mutational burden. Knockdown of RECQL4 inhibits cervical cancer cell proliferation, migration, and invasion, suppresses epithelial-mesenchymal transition status, induces cell cycle arrest, and promotes apoptosis. Mechanistically, RECQL4 mediated malignancy through the PI3K/AKT pathway and reduced nuclear β-catenin expression. In vivo studies further confirmed that RECQL4 knockout significantly inhibited tumor growth.
Conclusions
Our findings provide novel insights into the mechanism behind RECQL4-mediated cervical cancer progression through the PI3K/AKT pathway. Furthermore, our study suggests potential therapeutic strategies for targeting RECQL4 in cervical cancer treatment.
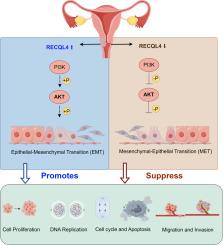
揭示 RECQL4 通过 PI3K/AKT 通路介导宫颈癌进展的机制。
背景RECQL4是DNA螺旋酶家族的成员,对DNA复制、DNA损伤修复和肿瘤进展至关重要。然而,它在宫颈癌中的具体作用仍不确定:本研究旨在利用癌症基因组图谱(The Cancer Genome Atlas)中的临床标本,研究 RECQL4 对宫颈癌预后的影响。我们通过细胞计数试剂盒-8、EdU、集落形成、细胞周期分析、细胞凋亡、划痕和 Transwell 试验等多种实验方法评估了 RECQL4 的恶性影响。我们通过生物信息学分析、RNA 测序数据、聚合酶链反应(PCR)、Western 印迹和细胞免疫荧光实验,探索了 RECQL4 调控恶性肿瘤的机制。此外,我们还利用裸鼠皮下肿瘤模型验证了 RECQL4 基因敲除对肿瘤生长的影响:结果:RECQL4在宫颈癌中上调,并与预后相关,与肿瘤突变负荷呈正相关。敲除 RECQL4 可抑制宫颈癌细胞的增殖、迁移和侵袭,抑制上皮-间质转化状态,诱导细胞周期停滞,促进细胞凋亡。从机理上讲,RECQL4通过PI3K/AKT途径介导恶性程度,并减少核β-catenin的表达。体内研究进一步证实,RECQL4敲除可显著抑制肿瘤生长:我们的研究结果为了解 RECQL4 通过 PI3K/AKT 通路介导宫颈癌进展的机制提供了新见解。此外,我们的研究还提出了在宫颈癌治疗中靶向 RECQL4 的潜在治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Translational Oncology
ONCOLOGY-
CiteScore
8.40
自引率
2.00%
发文量
314
审稿时长
54 days
期刊介绍:
Translational Oncology publishes the results of novel research investigations which bridge the laboratory and clinical settings including risk assessment, cellular and molecular characterization, prevention, detection, diagnosis and treatment of human cancers with the overall goal of improving the clinical care of oncology patients. Translational Oncology will publish laboratory studies of novel therapeutic interventions as well as clinical trials which evaluate new treatment paradigms for cancer. Peer reviewed manuscript types include Original Reports, Reviews and Editorials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


