Chronic alcohol consumption aggravates acute kidney injury through integrin β1/JNK signaling
IF 10.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Alcohol abuse is one of the major public health problems in the world and is associated with various health conditions. However, little is known about the effect of alcohol consumption on acute kidney injury (AKI). In this study, we demonstrate that chronic and binge alcohol feeding with a Lieber-DeCarli diet containing 5 % ethanol for 10 days, followed by a single dose of 31.5 % ethanol by gavage, aggravated AKI after ischemia-reperfusion injury (IRI) in female, but not in male, mice. Kidney dysfunction, histopathology and tubular cell apoptosis were more severe in EtOH-fed female mice after IRI, compared to pair-fed controls. RNA sequencing and experimental validation uncovered that activation of integrin β1 and its downstream c-Jun NH2-terminal kinase (JNK) aggravated AKI in EtOH-fed mice. Knockdown of integrin β1 inhibited JNK phosphorylation and alleviated AKI in EtOH-fed mice, whereas activation of integrin β1 by agonist antibody increased JNK phosphorylation, worsened renal histological injury and tubular cell apoptosis, and aggravated kidney dysfunction. In vitro, activation of integrin β1 increased JNK phosphorylation and induced tubular epithelial cell apoptosis. The detrimental effect of EtOH feeding was primarily mediated by acetaldehyde, as its levels were increased in the blood, liver and kidney of female mice fed with ethanol. Acetaldehyde per se activated integrin β1/JNK signaling and induced tubular cell apoptosis in vitro. These findings suggest that alcohol consumption increases vulnerability to AKI in female mice, which is probably mediated by acetaldehyde/integrin β1/JNK signaling cascade.
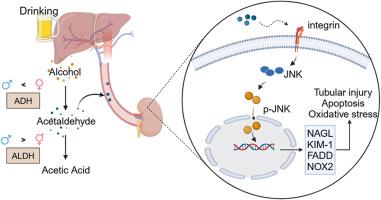
长期饮酒会通过整合素β1/JNK信号转导加重急性肾损伤。
酗酒是世界上主要的公共卫生问题之一,与各种健康状况有关。然而,人们对饮酒对急性肾损伤(AKI)的影响知之甚少。在这项研究中,我们证明了用含 5% 乙醇的 Lieber-DeCarli 饮料连续 10 天喂养雌性小鼠(而不是雄性小鼠)慢性和暴饮暴食酒精,然后单剂量灌胃 31.5% 的乙醇,会加重小鼠缺血再灌注损伤(IRI)后的急性肾损伤(AKI)。与对饲对照组相比,饲喂乙醇的雌性小鼠在缺血再灌注损伤后的肾功能障碍、组织病理学和肾小管细胞凋亡更为严重。RNA测序和实验验证发现,整合素β1及其下游的c-Jun NH2-末端激酶(JNK)的激活加重了EtOH喂养小鼠的AKI。敲除整合素β1可抑制JNK磷酸化并缓解EtOH喂养小鼠的AKI,而通过激动剂抗体激活整合素β1可增加JNK磷酸化,加重肾组织学损伤和肾小管细胞凋亡,并加剧肾功能障碍。在体外,激活整合素β1会增加JNK磷酸化,诱导肾小管上皮细胞凋亡。喂食乙醇的有害影响主要由乙醛介导,因为乙醛在喂食乙醇的雌性小鼠的血液、肝脏和肾脏中含量增加。乙醛本身会激活整合素β1/JNK信号传导,诱导体外肾小管细胞凋亡。这些发现表明,饮酒会增加雌性小鼠对 AKI 的易感性,这可能是由乙醛/整合素 β1/JNK 信号级联介导的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


