NSUN2 regulates Wnt signaling pathway depending on the m5C RNA modification to promote the progression of hepatocellular carcinoma
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
5-Methylcytosine (m5C) RNA modification is a highly abundant and important epigenetic modification in mammals. As an important RNA m5C methyltransferase, NOP2/Sun-domain family member 2 (NSUN2)-mediated m5C RNA modification plays an important role in the regulation of the biological functions in many cancers. However, little is known about the biological role of NSUN2 in hepatocellular carcinoma (HCC). In this study, we found that the expression of NSUN2 was significantly upregulated in HCC, and the HCC patients with higher expression of NSUN2 had a poorer prognosis than those with lower expression of NSUN2. NSUN2 could affect the tumor immune regulation of HCC in several ways. In vitro and in vivo experiments confirmed that NSUN2 knockdown significantly decreased the abilities of proliferation, colony formation, migration and invasion of HCC cells. The methylated RNA immunoprecipitation-sequencing (MeRIP-seq) showed NSUN2 knockdown significantly affected the abundance, distribution, and composition of m5C RNA modification in HCC cells. Functional enrichment analyses and in vitro experiments suggested that NSUN2 could promote the HCC cells to proliferate, migrate and invade by regulating Wnt signaling pathway. SARS2 were identified via the RNA immunoprecipitation-sequencing (RIP-Seq) and MeRIP-seq as downstream target of NSUN2, which may play an important role in tumor-promoting effect of NSUN2-mediated m5C RNA modification in HCC. In conclusion, NSUN2 promotes HCC progression by regulating Wnt signaling pathway and SARS2 in an m5C-dependent manner.
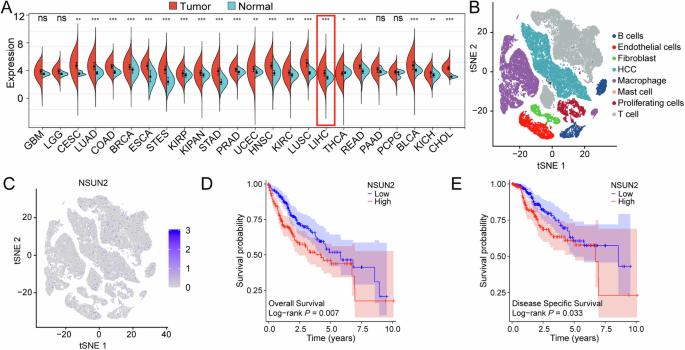
NSUN2 依靠 m5C RNA 修饰调控 Wnt 信号通路,促进肝细胞癌的进展。
5-甲基胞嘧啶(m5C)RNA修饰是哺乳动物体内一种高度丰富和重要的表观遗传修饰。作为一种重要的 RNA m5C 甲基转移酶,NOP2/Sun-domain 家族成员 2(NSUN2)介导的 m5C RNA 修饰在许多癌症的生物功能调控中发挥着重要作用。然而,人们对NSUN2在肝细胞癌(HCC)中的生物学作用知之甚少。本研究发现,NSUN2在HCC中的表达明显上调,且NSUN2表达较高的HCC患者的预后比NSUN2表达较低的患者差。NSUN2可通过多种途径影响HCC的肿瘤免疫调节。体外和体内实验证实,NSUN2基因敲除可显著降低HCC细胞的增殖、集落形成、迁移和侵袭能力。甲基化RNA免疫沉淀测序(MeRIP-sequencing)显示,NSUN2基因敲除明显影响了HCC细胞中m5C RNA修饰的丰度、分布和组成。功能富集分析和体外实验表明,NSUN2可通过调控Wnt信号通路促进HCC细胞的增殖、迁移和侵袭。通过RNA免疫沉淀测序(RIP-Seq)和MeRIP-seq发现,SARS2是NSUN2的下游靶点,可能在NSUN2介导的m5C RNA修饰对HCC的促瘤效应中发挥重要作用。总之,NSUN2通过调控Wnt信号通路和SARS2以m5C依赖的方式促进HCC的进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


