Inhibition and degradation of NRAS with a pan-NRAS monobody
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The RAS family GTPases are the most frequently mutated oncogene family in human cancers. Activating mutations in either of the three RAS isoforms (HRAS, KRAS, or NRAS) are found in nearly 20% of all human tumors with NRAS mutated in ~25% of melanomas. Despite remarkable advancements in therapies targeted against mutant KRAS, NRAS-specific pharmacologics are lacking. Thus, development of inhibitors of NRAS would address a critical unmet need to treating primary tumors harboring NRAS mutations as well as BRAF-mutant melanomas, which frequently develop resistance to clinically approved BRAF inhibitors through NRAS mutation. Building upon our previous studies with the monobody NS1 that recognizes HRAS and KRAS but not NRAS, here we report the development of a monobody that specifically binds to both GDP and GTP-bound states of NRAS and inhibits NRAS-mediated signaling in a mutation-agnostic manner. Further, this monobody can be formatted into a genetically encoded NRAS-specific degrader. Our study highlights the feasibility of developing NRAS selective inhibitors for therapeutic efforts.
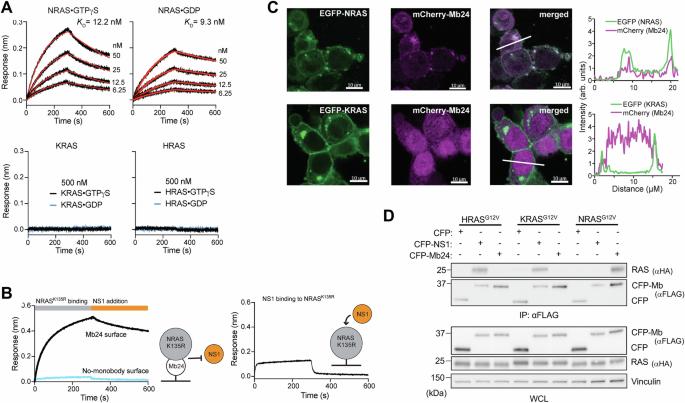
用泛 NRAS 单体抑制和降解 NRAS。
RAS 家族 GTP 酶是人类癌症中最常发生突变的癌基因家族。三种 RAS 异构体(HRAS、KRAS 或 NRAS)的激活突变在近 20% 的人类肿瘤中均有发现,而在约 25% 的黑色素瘤中发现了 NRAS 突变。尽管针对突变 KRAS 的疗法取得了重大进展,但仍缺乏 NRAS 特异性药物。因此,NRAS 抑制剂的开发将满足治疗 NRAS 突变原发肿瘤和 BRAF 突变黑色素瘤的关键需求。我们之前研究的单体 NS1 能识别 HRAS 和 KRAS,但不能识别 NRAS,在此我们报告了我们开发的一种单体,它能特异性地与 NRAS 的 GDP 和 GTP 结合态结合,并以突变无关的方式抑制 NRAS 介导的信号转导。此外,这种单体还可以格式化为基因编码的 NRAS 特异性降解器。我们的研究强调了开发用于治疗的 NRAS 选择性抑制剂的可行性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


