Silencing of SIRPα enhances the antitumor efficacy of CAR-M in solid tumors
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
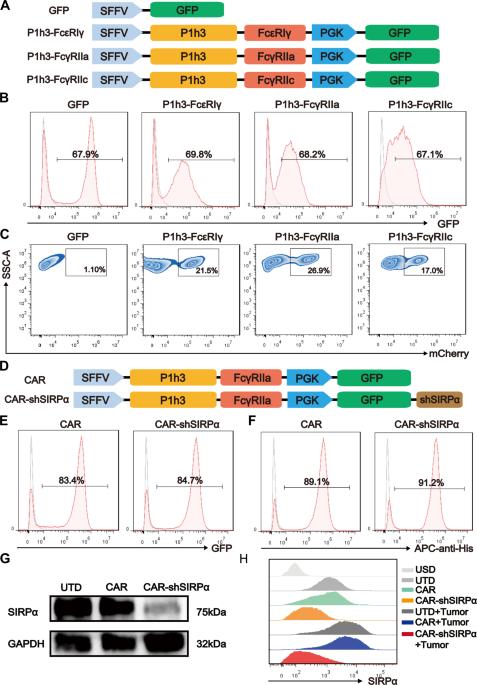
The potential of macrophage-mediated phagocytosis as a cancer treatment is promising. Blocking the CD47–SIRPα interaction with a CD47-specific antibody significantly enhances macrophage phagocytosis. However, concerns regarding their toxicity to nontumor cells remain substantial. Here, we engineered chimeric antigen receptor macrophages (CAR-Ms) by fusing a humanized single-chain variable fragment with FcγRIIa and integrating short hairpin RNA to silence SIRPα, thereby disrupting the CD47–SIRPα signaling pathway. These modified CAR-shSIRPα-M cells exhibited an M1-like phenotype, superior phagocytic function, substantial cytotoxic effects on HER2-positive tumor cells, and the ability to eliminate patient-derived organoids. In vivo, CAR-M cells significantly inhibited tumor growth and prolonged survival in tumor-bearing mice. Notably, CAR-shSIRPα-M cells enhanced cytotoxic T-cell infiltration into tumors, thereby enhancing the antitumor response in both the humanized immune system mouse model and immunocompetent mice. Mechanistically, SIRPα inhibition activated inflammatory pathways and the cGAS-STING signaling cascade in CAR-M cells, leading to increased production of proinflammatory cytokines, reactive oxygen species, and nitric oxide, thereby enhancing their antitumor effects. These findings underscore the potential of SIRPα inhibition as a novel strategy to increase the antitumor efficacy of CAR-M cells in cancer immunotherapy, particularly against solid tumors.
沉默 SIRPα 可增强 CAR-M 在实体瘤中的抗肿瘤疗效。
巨噬细胞介导的吞噬作用作为癌症治疗手段的潜力令人期待。用CD47特异性抗体阻断CD47-SIRPα相互作用可显著增强巨噬细胞的吞噬能力。然而,它们对非肿瘤细胞的毒性仍然令人担忧。在这里,我们通过将人源化单链可变片段与 FcγRIIa 融合并整合短发夹 RNA 来抑制 SIRPα,从而破坏 CD47-SIRPα 信号通路,设计出嵌合抗原受体巨噬细胞(CAR-Ms)。这些经过修饰的CAR-shSIRPα-M细胞表现出M1样表型、卓越的吞噬功能、对HER2阳性肿瘤细胞的巨大细胞毒性作用以及消除患者衍生器官组织的能力。在体内,CAR-M 细胞能显著抑制肿瘤生长,延长肿瘤小鼠的存活时间。值得注意的是,CAR-shSIRPα-M 细胞增强了细胞毒性 T 细胞对肿瘤的浸润,从而增强了人源化免疫系统小鼠模型和免疫功能健全小鼠的抗肿瘤反应。从机理上讲,SIRPα抑制激活了CAR-M细胞的炎症通路和cGAS-STING信号级联,导致促炎细胞因子、活性氧和一氧化氮的产生增加,从而增强了它们的抗肿瘤作用。这些发现强调了抑制 SIRPα 作为一种新策略的潜力,可提高 CAR-M 细胞在癌症免疫疗法中的抗肿瘤疗效,尤其是对实体瘤的疗效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


