Whole tumour- and subregion-based radiomics of contrast-enhanced mammography in differentiating HER2 expression status of invasive breast cancers: A double-centre pilot study
IF 6.4
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
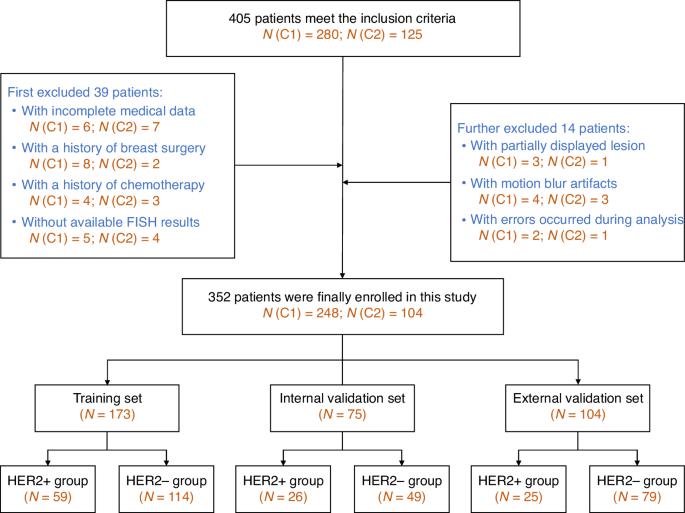
To explore the value of whole tumour- and subregion-based radiomics of contrast-enhanced mammography (CEM) in differentiating the HER2 expression status of breast cancers. 352 patients underwent preoperative CEM from two centres were consecutively enroled and divided into the training, internal validation, and external validation cohorts. The lesions were divided into HER2-positive and HER2-negative groups. Besides the radiological features, radiomics features capturing the whole tumour-based (wITH) and subregion-based intratumoral heterogeneity (sITH) were extracted from the craniocaudal view of CEM recombined images. The XGBoost classifier was applied to develop the radiological, sITH, and wITH models. A combined model was constructed by fusing the prediction results of the three models. The mean age of the patients was 51.1 ± 10.7 years. Two radiological features, four wITH features, and three sITH features were selected to establish the models. The combined model significantly improved the AUC to 0.80 ± 0.03 (95% CI: 0.73–0.86), 0.79 ± 0.06 (95% CI: 0.67–0.90), and 0.79 ± 0.05 (95% CI: 0.69–0.89) in the training, internal validation, and external validation cohorts, respectively (All P < 0.05). The combined model showed good agreement between the predicted and observed probabilities and favourable net clinical benefit in the validation cohorts. Both whole tumour- and subregion-based ITH radiomics features of CEM exhibited potential for differentiating the HER2 expression status. Combining conventional radiological features and ITH features can improve the model’s performance.
基于全肿瘤和亚区域的放射组学对比增强乳腺 X 射线造影在区分浸润性乳腺癌 HER2 表达状态方面的应用:一项双中心试点研究。
目的:探讨对比增强乳腺X线摄影术(CEM)基于全肿瘤和亚区域的放射组学在区分乳腺癌HER2表达状态方面的价值:方法:连续登记两个中心接受术前CEM检查的352例患者,并将其分为训练组、内部验证组和外部验证组。病变分为 HER2 阳性组和 HER2 阴性组。除了放射学特征外,还从 CEM 重组图像的颅尾视图中提取了捕捉基于整个肿瘤(wITH)和基于亚区域的瘤内异质性(sITH)的放射组学特征。XGBoost 分类器用于开发放射学模型、sITH 模型和 wITH 模型。通过融合三个模型的预测结果,构建了一个综合模型:患者的平均年龄为 51.1 ± 10.7 岁。建立模型时选择了两个放射学特征、四个 wITH 特征和三个 sITH 特征。在训练组、内部验证组和外部验证组中,组合模型的AUC分别为0.80±0.03(95% CI:0.73-0.86)、0.79±0.06(95% CI:0.67-0.90)和0.79±0.05(95% CI:0.69-0.89)(所有P均为结论),明显提高了AUC:基于全肿瘤和亚区域的CEM ITH放射组学特征都具有区分HER2表达状态的潜力。结合常规放射学特征和 ITH 特征可以提高模型的性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

British Journal of Cancer
医学-肿瘤学
CiteScore
15.10
自引率
1.10%
发文量
383
审稿时长
6 months
期刊介绍:
The British Journal of Cancer is one of the most-cited general cancer journals, publishing significant advances in translational and clinical cancer research.It also publishes high-quality reviews and thought-provoking comment on all aspects of cancer prevention,diagnosis and treatment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


