Synthesis, structures, and cytotoxicity insights of organotin(IV) complexes with thiazole-appended pincer ligand
IF 3.8
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Diorganotin complexes of the compositions [Me2Sn(L)] (1), [n-Bu2Sn(L)] (2), [Ph2Sn(L)]⋅C6H6 (3), [Bz2Sn(L)]⋅C6H6 (4) and [n-Oct2Sn(L)] (5) were synthesized by reacting R2SnO (R = Me, n-Bu, Ph, Bz or n-Oct) with the N2,N6-di(thiazol-2-yl)pyridine-2,6-dicarboxamide (H2L, where H2 denotes the two acidic protons) in refluxing toluene. Additionally, the mono-n-butyltin complex [n-BuSn(HL)Cl2]·H2O (6) was synthesized from n-BuSnCl3 and H2L in acetonitrile. Compounds were characterized by FT-IR, 1H, 13C and 119Sn NMR spectroscopy, while their solid-state structures were examined using single-crystal X-ray diffraction studies. In diorganotin compounds 1–5, the dianionic tridentate ligands (Npy, N−, N−) act as κ-N3 chelators. In 6, the L moiety (O, Npy, N−) acts as a κ-ON2 tridentate chelator, with involvement of one of the carboxamide oxygen atoms. The coordination polyhedron around the Sn(IV) ion is completed either by two axial Sn-R ligands in compounds 1–5 or by n-Bu and Cl ligands in compound 6, giving rise to distorted trigonal bipyramid or octahedral structures, respectively. The tin NMR results show that the penta-coordinated structures of compounds 1–5 and the hexacoordinated structure of compound 6, observed in the solid-state, are retained in solution. The in vitro antitumor activities of 1–5 were tested on T-47D breast cancer cells. Of these, diphenyltin compound 3 showed the highest anti-proliferative effect, with an IC50 of 10 ± 1.60 μM. Compound 3 exhibited selective toxicity, potentially inducing apoptosis via reactive oxygen species generation and nuclear changes, indicating promise as a breast cancer treatment. This study is the first to explore thiazole-appended organotin compounds for cytotoxicity.
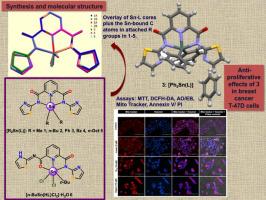
有机锡(IV)与噻唑添加钳配体配合物的合成、结构和细胞毒性研究。
通过将 R2SnO(R = Me、n-Bu、Ph、Bz 或 n-Oct)与 N2,N6-二(噻唑-2-基)吡啶-2,6-二甲酰胺(H2L,其中 H2 表示两个酸性质子)在回流甲苯中反应而合成。此外,正丁基锡化合物[n-BuSn(HL)Cl2]-H2O(6)也是由正丁基锡化合物 H2L 在乙腈中合成的。化合物的表征采用了傅立叶变换红外光谱、1H、13C 和 119Sn NMR 光谱,其固态结构则采用了单晶 X 射线衍射研究。在二氧化甘油锡化合物 1-5 中,二阴离子三叉配体(Npy、N-、N-)起着κ-N3 螯合剂的作用。在 6 中,L 离子(O、Npy、N-)作为κ-ON2 三叉螯合剂,其中一个羧酰胺氧原子参与其中。围绕 Sn(IV)离子的配位多面体在化合物 1-5 中由两个轴向 Sn-R 配体完成,或在化合物 6 中由 n-Bu 和 Cl 配体完成,分别形成扭曲的三叉二面体或八面体结构。锡核磁共振结果表明,固态下观察到的化合物 1-5 的五配位结构和化合物 6 的六配位结构在溶液中得以保留。在 T-47D 乳腺癌细胞上测试了 1-5 化合物的体外抗肿瘤活性。其中,二苯基锡化合物 3 的抗增殖作用最强,IC50 为 10 ± 1.60 μM。化合物 3 显示出选择性毒性,可能通过活性氧生成和核变化诱导细胞凋亡,显示出治疗乳腺癌的前景。这项研究首次探索了噻唑类有机锡化合物的细胞毒性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


