Synthesis, optimization and antitumor activity evaluation of sulfonyl benzoyl hydrazide derivatives as novel human LSD1 inhibitors
IF 2.5
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
A new set of compounds known as sulfonyl benzoyl hydrazide derivatives were synthesized and tested using cellular assays. Through systematic optimization starting from general structure S-1, compound 10e emerged as highly promising. It exhibited potent inhibitory activity with an IC50 value of 0.8 nM and possessed moderate clogP. Compounds 10e significantly inhibited solid tumor cells proliferation. Additionally, 10e induced apoptosis and arrested the cell cycle. Furthermore, in vivo studies using an HCT116 xenograft model showed substantial growth inhibition of tumors, accompanied by a favorable safety profile. These findings underscored compound 10e as a novel LSD1 inhibitor with robust efficacy both in vitro and in vivo, establishing it as a promising lead compound for further anticancer drug development.
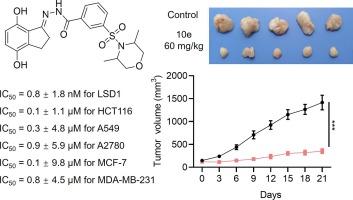
磺酰基苯甲酰肼衍生物作为新型人类 LSD1 抑制剂的合成、优化和抗肿瘤活性评价。
我们合成了一组名为磺酰基苯甲酰肼衍生物的新化合物,并使用细胞检测法进行了测试。通过从一般结构 S-1 开始的系统优化,化合物 10e 成为极具潜力的化合物。它具有强效的抑制活性,IC50 值为 0.8 nM,并具有适度的 ClogP。化合物 10e 能明显抑制实体瘤细胞的增殖。此外,10e 还能诱导细胞凋亡并阻滞细胞周期。此外,使用 HCT116 异种移植模型进行的体内研究显示,化合物 10e 可大幅抑制肿瘤生长,同时具有良好的安全性。这些研究结果表明,化合物 10e 是一种新型 LSD1 抑制剂,在体外和体内都有很强的疗效,有望成为进一步开发抗癌药物的先导化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


