Design, synthesis and biological evaluation of novel benzocoumarin derivatives as potent inhibitors of MAO-B activity
IF 2.5
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
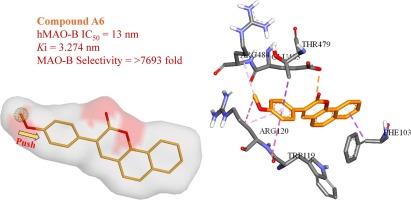
The continued research of novel reversible inhibitors targeting monoamine oxidase (MAO) B remains crucial for effectively symptomatic treatment of Parkinson’s disease. In this study we synthesized and evaluated a new series of 3-aryl benzo[g] and benzo[h] coumarin derivatives as MAO-B inhibitors. Compound A6 has been found to display the most potent inhibitory activity and selectivity against the MAO-B isoform (IC50 = 13 nM and SI = >7693.31 respectively). Inhibition mode of A6 on MAO-B was predicted as mixed reversible inhibition with a Ki value of 3.274 nM. Furthermore, in order to elaborate structure–activity relationships, the binding mode of A6 was investigated by molecular docking simulations.
新型苯并香豆素衍生物作为 MAO-B 活性强效抑制剂的设计、合成和生物学评价。
针对单胺氧化酶(MAO)B 的新型可逆抑制剂的持续研究对于帕金森病的有效对症治疗仍然至关重要。在这项研究中,我们合成并评估了一系列新的 3-芳基苯并[g]和苯并[h]香豆素衍生物作为 MAO-B 抑制剂。研究发现,化合物 A6 对 MAO-B 同工酶具有最强的抑制活性和选择性(IC50 = 13 nM 和 SI = >7693.31)。根据预测,A6 对 MAO-B 的抑制模式为混合可逆抑制,Ki 值为 3.274 nM。此外,为了阐述结构-活性关系,还通过分子对接模拟研究了 A6 的结合模式。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


