Photoredox Oxidation of Alkanes by Monometallic Copper–Oxygen Complexes Using Visible Light Including One Sun Illumination
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Oxygenation of hydrocarbons offers versatile catalytic routes to more valuable compounds, such as alcohols, aldehydes, and ketones. Despite the importance of monometallic copper–oxygen species as hydroxylating agents in biology, few synthetic model compounds are known to react with hydrocarbons, owing to high C–H bond dissociation energies. To overcome this challenge, the photoredox chemistry of monometallic copper (pyrazolyl)borate complexes coordinated by chlorate has been explored in the presence of C1–C6 alkanes with BDEs ≥ 93 kcal/mol. Ethane is photooxidized at room temperature under N2 with yields of 15–30%, which increases to 77% for the most oxidizing tris(3,5-trifluoromethyl-pyrazolyl)borate complex (Cu-3). This complex also promotes the photooxidation of methane to methanol in significant yield (38%) when the photoredox reaction is run under aerobic conditions. Ligand modification alters the reaction selectivity by tuning the redox potential. The ability to activate 1° C–H bonds of C1–C6 alkanes using visible light is consistent with the photogeneration of a powerfully oxidizing copper-oxyl, which is supported by photocrystallographic studies of the tris(3,4,5-tribromopyrazolyl)borate chlorate complex. Mechanistic studies are consistent with the hydrogen atom abstraction of the C–H bond by the copper-oxyl intermediate. We demonstrate for Cu-3 with hexane as an exemplar, that the photoredox chemistry may be achieved under solar conditions of one-sun illumination.
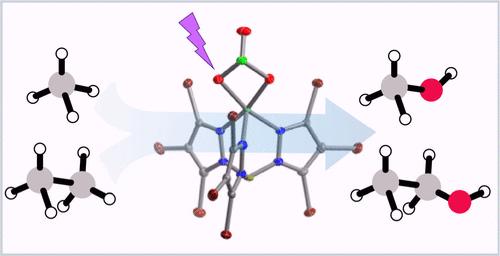
单金属铜氧配合物利用可见光(包括一个太阳的光照)对烷烃的光氧化反应
碳氢化合物的氧合反应为获得更有价值的化合物(如醇、醛和酮)提供了多种催化途径。尽管单金属铜氧物种作为羟化剂在生物学中非常重要,但由于 C-H 键解离能较高,很少有合成模型化合物能与碳氢化合物发生反应。为了克服这一挑战,研究人员在 BDE ≥ 93 kcal/mol 的 C1-C6 烷烃存在下,探索了由氯酸盐配位的单金属铜(吡唑基)硼酸盐配合物的光氧化化学性质。乙烷在室温、氮气条件下发生光氧化反应的产率为 15-30%,而氧化性最强的三(3,5-三氟甲基吡唑基)硼酸酯络合物(Cu-3)的产率增至 77%。当光氧化反应在有氧条件下进行时,该配合物还能促进甲烷光氧化成甲醇,产率可观(38%)。配体修饰通过调整氧化还原电位来改变反应的选择性。利用可见光激活 C1-C6 烷烃的 1° C-H 键的能力与光生成强氧化性铜氧基体的能力是一致的,这一点得到了三(3,4,5-三溴吡唑基)硼酸盐氯酸盐复合物的光晶体学研究的支持。机理研究与铜氧中间体抽取 C-H 键的氢原子相一致。我们以 Cu-3 和正己烷为例,证明了光氧化化学反应可以在一太阳光照条件下实现。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


