A Kinetic Trapping Approach for Facile Access to 3FaxNeu5Ac and a Photo-Cross-Linkable Sialyltransferase Probe
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
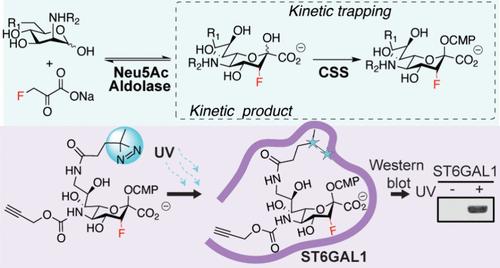
Sialic acid (Neu5Ac) is installed onto glycoconjugates by sialyltransferases (STs) using cytidine monophosphate-Neu5Ac (CMP-β-d-Neu5Ac) as their donor. The only class of cell-active ST inhibitors are those based on a 3FaxNeu5Ac scaffold, which is metabolically converted into CMP-3FaxNeu5Ac within cells. It is essential for the fluorine to be axial, yet stereoselective installation of fluorine in this specific orientation is challenging. Sialic acid aldolase can convert 3-fluoropyruvate and 2-acetamido-2-deoxy-d-mannopyranose (ManNAc) to 3FNeu5Ac, but stereocontrol of the fluorine in the product has not been possible. We hypothesized that the 3Fax kinetic product of a sialic acid aldolase reaction could be trapped by coupling with CMP-sialic acid synthetase to yield CMP-3FaxNeu5Ac. Here, we report that highly active CMP-sialic acid synthetase and short reaction times produce exclusively CMP-3FaxNeu5Ac. Removal of CMP from CMP-3FaxNeu5Ac under acidic conditions unexpectedly led to 3-fluoro-β-d-Neu5Ac 2-phosphate (3FaxNeu5Ac-2P). Alkaline phosphatase successfully converted 3FaxNeu5Ac-2P to 3FaxNeu5Ac, enabling stereochemically controlled access to 3FaxNeu5Ac, which is effective in lowering the sialoglycan ligands for Siglecs on cells. Moreover, our kinetic trapping approach could be used to access CMP-3FaxNeu5Ac with modifications at the C5, C9, or both positions, which enabled the chemoenzymatic synthesis of a photo-cross-linkable version of CMP-3FaxNeu5Ac that selectively photo-cross-linked to ST6GAL1 over two other STs.
便捷获取 3FaxNeu5Ac 和光交联硅氨基转移酶探针的动力学诱捕方法
以单磷酸胞苷-Neu5Ac(CMP-β-d-Neu5Ac)为供体的硅氨酰转移酶(ST)将硅氨酰酸(Neu5Ac)安装到糖共轭物上。唯一一类具有细胞活性的 ST 抑制剂是基于 3FaxNeu5Ac 支架的抑制剂,这种支架会在细胞内代谢转化为 CMP-3FaxNeu5Ac。氟必须是轴向的,但要立体选择性地将氟安装在这一特定方向却很困难。唾液酸醛缩酶可将 3-氟丙酮酸和 2-乙酰氨基-2-脱氧-d-吡喃甘露糖(ManNAc)转化为 3FNeu5Ac,但还无法对产物中的氟进行立体控制。我们假设,通过与 CMP-硅烷酸合成酶偶联生成 CMP-3FaxNeu5Ac,可以捕获硅烷酸醛缩酶反应的 3Fax 动力产物。在这里,我们报告了高活性 CMP-半乳糖醛酸合成酶和短反应时间只产生 CMP-3FaxNeu5Ac。在酸性条件下去除 CMP-3FaxNeu5Ac 中的 CMP,意外地生成了 3-氟-β-d-Neu5Ac 2-磷酸(3FaxNeu5Ac-2P)。碱性磷酸酶成功地将 3FaxNeu5Ac-2P 转化为 3FaxNeu5Ac,实现了立体化学控制的 3FaxNeu5Ac 的获取,从而有效地降低了细胞上 Siglecs 的sialoglycan 配体。此外,我们的动力学诱捕方法还可用于获得在 C5、C9 或两个位置上都进行了修饰的 CMP-3FaxNeu5Ac,这使得化学酶法合成的光交联型 CMP-3FaxNeu5Ac 能选择性地与 ST6GAL1 光交联,而不是与其他两种 ST 光交联。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


