Direct access to thiomethyl/selenomethyl-substituted pyrazoles by combining isocyanide insertion into the inert C(sp)–S bond and intermolecular cyclization †
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
In this study, we present a facile and efficient one-pot synthetic protocol for the construction of thiomethyl/selenomethyl substituted pyrazoles. This protocol involves palladium-catalyzed isocyanide insertion into the C(sp)–S bond, followed by TMSN3-involved CuI/Sc(OTf)3 catalyzed intermolecular cyclization. The novel reaction of isocyanide insertion into the C(sp)–S bond achieves high atom economy while preserving the integrity of the CC bond. The subsequent cyclization process entails an intermolecular reaction between an alkynyl imine and TMSN3, with the alkynyl imine playing a crucial role as an intermediate in the reaction pathway.
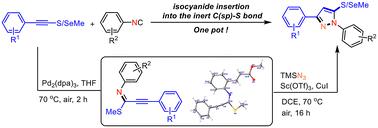
通过异氰酸酯插入惰性 C(sp)-S 键和分子间环化作用,直接获得硫代甲基/硒代甲基吡唑
在本研究中,我们提出了一种简便高效的单锅合成方案,用于构建硫代甲基/硒代甲基取代的吡唑。该方案包括在钯催化下将异氰酸酯插入 C(sp)-S 键,然后在 TMSN3 参与的 CuI/Sc(OTf)3 催化下进行分子间环化。异氰酸酯插入 C(sp)-S 键的新反应实现了高原子经济性,同时保持了 C≡C 键的完整性。随后的环化过程涉及炔基亚胺和 TMSN3 之间的分子间反应,其中炔基亚胺在反应途径中起着关键的中间体作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


