HOXDeRNA activates a cancerous transcription program and super enhancers via genome-wide binding
IF 16.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The role of long non-coding RNAs (lncRNAs) in malignant cell transformation remains elusive. We previously identified an enhancer-associated lncRNA, LINC01116 (named HOXDeRNA), as a transformative factor converting human astrocytes into glioma-like cells. Employing a combination of CRISPR editing, chromatin isolation by RNA purification coupled with sequencing (ChIRP-seq), in situ mapping RNA-genome interactions (iMARGI), chromatin immunoprecipitation sequencing (ChIP-seq), HiC, and RNA/DNA FISH, we found that HOXDeRNA directly binds to CpG islands within the promoters of 35 glioma-specific transcription factors (TFs) distributed throughout the genome, including key stem cell TFs SOX2, OLIG2, POU3F2, and ASCL1, liberating them from PRC2 repression. This process requires a distinct RNA quadruplex structure and other segments of HOXDeRNA, interacting with EZH2 and CpGs, respectively. Subsequent transformation activates multiple oncogenes (e.g., EGFR, miR-21, and WEE1), driven by the SOX2- and OLIG2-dependent glioma-specific super enhancers. These results help reconstruct the sequence of events underlying the process of astrocyte transformation, highlighting HOXDeRNA’s central genome-wide activity and suggesting a shared RNA-dependent mechanism in otherwise heterogeneous and multifactorial gliomagenesis.
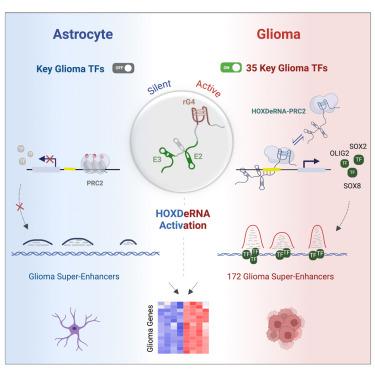
HOXDeRNA 通过全基因组结合激活癌症转录程序和超级增强子
长非编码 RNA(lncRNA)在恶性细胞转化中的作用仍然难以捉摸。我们之前发现了一种增强子相关的lncRNA--LINC01116(命名为HOXDeRNA),它是将人类星形胶质细胞转化为胶质瘤样细胞的转化因子。我们结合使用了 CRISPR 编辑、染色质分离 RNA 纯化测序(ChIRP-seq)、原位绘制 RNA 基因组相互作用(iMARGI)、染色质免疫沉淀测序(ChIP-seq)、HiC 和 RNA/DNA FISH、我们发现,HOXDeRNA直接与分布在整个基因组的35个胶质瘤特异性转录因子(TFs)启动子内的CpG岛结合,包括关键的干细胞TFs SOX2、OLIG2、POU3F2和ASCL1,使它们摆脱PRC2的抑制。这一过程需要独特的 RNA 四重结构和 HOXDeRNA 的其他片段,它们分别与 EZH2 和 CpGs 相互作用。随后的转化会激活多种癌基因(如表皮生长因子受体、miR-21 和 WEE1),并由 SOX2- 和 OLIG2 依赖性胶质瘤特异性超级增强子驱动。这些结果有助于重构星形胶质细胞转化过程的事件序列,突出了 HOXDeRNA 在全基因组中的核心活性,并表明在其他异质性和多因素的胶质瘤发生过程中存在一种共享的 RNA 依赖性机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


