Notch-Driven Cholangiocarcinogenesis Involves the Hippo Pathway Effector TAZ via METTL3-m6A-YTHDF1
IF 7.1
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
Cellular and Molecular Gastroenterology and Hepatology
Pub Date : 2024-10-05
DOI:10.1016/j.jcmgh.2024.101417
引用次数: 0
Abstract
Background & Aims
Notch and TAZ are implicated in cholangiocarcinogenesis, but whether and how these oncogenic molecules interact remain unknown.
Methods
The development of cholangiocarcinoma (CCA) was induced by hydrodynamic tail vein injection of oncogenes (Notch1 intracellular domain [NICD]/AKT) to the FVB/NJ mice. CCA xenograft was developed by inoculation of human CCA cells into the livers of SCID mice. Tissues and cells were analyzed using quantitative reverse transcription polymerase chain reaction, Western blotting analyses, immunohistochemistry, chromatin immunoprecipitation-quantitative polymerase chain reaction and WST-1 cell proliferation assay.
Results
Our experimental findings show that TAZ is indispensable in NICD-driven cholangiocarcinogenesis. Notch activation induces the expression of methyltransferase like-3 (METTL3), which catalyzes N6-methyladenosine modification of TAZ mRNA and that this mechanism plays a central role in the crosstalk between Notch and TAZ in CCA cells. Mechanistically, Notch regulates the expression of METTL3 through the binding of NICD to its downstream transcription factor CSL in the promoter region of METTL3. METTL3 in turn mediates N6-methyladenosine modification of TAZ mRNA, which is recognized by the m6A reader YTHDF1 to enhance TAZ protein translation. We observed that inhibition of Notch signaling decreased the protein levels of both MELLT3 and TAZ. Depletion of METTL3 by short hairpin RNAs or by the next generation GapmeR antisense oligonucleotides decreased the level of TAZ protein and inhibited the growth of human CCA cells in vitro and in mice.
Conclusion
This study describes a novel Notch-METTL3-TAZ signaling cascade, which is important in CCA development and progression. Our experimental results provide new insight into how the Notch pathway cooperates with TAZ signaling in CCA, and the findings may have important therapeutic implications.
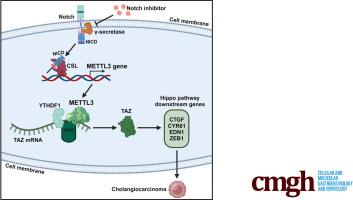
Notch驱动的胆管癌发生涉及通过METTL3-m6A-YTHDF1的Hippo通路效应因子TAZ
背景和目的:Notch和TAZ与胆管癌的发生有关,但这些致癌分子是否以及如何相互作用仍不清楚:方法:通过向 FVB/NJ 小鼠尾静脉注射致癌基因(NICD/AKT)诱导其发生 CCA。将人 CCA 细胞接种到 SCID 小鼠肝脏中,形成 CCA 异种移植。采用qRT-PCR、Western印迹分析、免疫组织化学、ChIP-qPCR和WST-1细胞增殖测定等方法对组织和细胞进行分析:我们的实验结果表明,TAZ 在 NICD 驱动的胆管癌发生过程中不可或缺。Notch激活可诱导METTL3(类似甲基转移酶-3)的表达,而METTL3可催化TAZ mRNA的N6-甲基腺苷(m6A)修饰,这一机制在CCA细胞中Notch与TAZ的相互影响中起着核心作用。从机制上讲,Notch通过NICD与其下游转录因子CSL在METTL3启动子区的结合来调节METTL3的表达。METTL3反过来又介导了TAZ mRNA的m6A修饰,这种修饰被m6A阅读器YTHDF1识别,从而增强了TAZ蛋白的翻译。我们观察到,抑制Notch信号传导会降低MELLT3和TAZ的蛋白水平。通过 shRNAs 或新一代 GapmeR 反义寡核苷酸(ASOs)去除 METTL3 可降低 TAZ 蛋白水平,并抑制体外和小鼠人 CCA 细胞的生长:本研究描述了一种新型的Notch-METTL3-TAZ信号级联,它在CCA的发生和发展过程中起着重要作用。我们的实验结果为了解 Notch 通路与 TAZ 信号在 CCA 中的合作提供了新的视角,这些发现可能具有重要的治疗意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cellular and Molecular Gastroenterology and Hepatology
Medicine-Gastroenterology
CiteScore
13.00
自引率
2.80%
发文量
246
审稿时长
42 days
期刊介绍:
"Cell and Molecular Gastroenterology and Hepatology (CMGH)" is a journal dedicated to advancing the understanding of digestive biology through impactful research that spans the spectrum of normal gastrointestinal, hepatic, and pancreatic functions, as well as their pathologies. The journal's mission is to publish high-quality, hypothesis-driven studies that offer mechanistic novelty and are methodologically robust, covering a wide range of themes in gastroenterology, hepatology, and pancreatology.
CMGH reports on the latest scientific advances in cell biology, immunology, physiology, microbiology, genetics, and neurobiology related to gastrointestinal, hepatobiliary, and pancreatic health and disease. The research published in CMGH is designed to address significant questions in the field, utilizing a variety of experimental approaches, including in vitro models, patient-derived tissues or cells, and animal models. This multifaceted approach enables the journal to contribute to both fundamental discoveries and their translation into clinical applications, ultimately aiming to improve patient care and treatment outcomes in digestive health.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


