Rational design, synthesis, and biophysical characterization of a peptidic MDM2-MDM4 interaction inhibitor
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
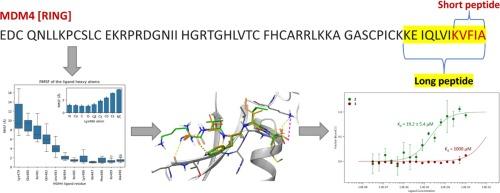
In recent years, the restoration of p53 physiological functions has become an attractive therapeutic approach to develop novel and efficacious cancer therapies. Among other mechanisms, the oncosuppressor protein p53 is functionally regulated by MDM2 through its E3 ligase function. MDM2 promotes p53 ubiquitination and degradation following homodimerization or heterodimerization with MDM4. Recently, we discovered Pep3 (1, Pellegrino et al., 2015), a novel peptidic inhibitor of MDM2 dimerization able to restore p53 oncosuppressive functions both in vitro and in vivo. In this work, we were able to identify the key interactions between peptide 1 and MDM2 RING domain and to design peptide 2, a truncated version of 1 that is still able to bind MDM2. Integrating both computational and biophysical techniques, we show that peptide 2 maintains the conserved peptide 1-MDM2 interactions and is still able to bind to full-length MDM2.
一种多肽 MDM2-MDM4 相互作用抑制剂的合理设计、合成和生物物理表征。
近年来,恢复 p53 的生理功能已成为开发新型高效癌症疗法的一种极具吸引力的治疗方法。除其他机制外,抑癌基因 p53 通过其 E3 连接酶功能受 MDM2 的功能调控。MDM2 与 MDM4 同二聚化或异二聚化后可促进 p53 泛素化和降解。最近,我们发现了 Pep3(1,Pellegrino 等人,2015 年),它是 MDM2 二聚化的一种新型多肽抑制剂,能够在体外和体内恢复 p53 的抑制功能。在这项工作中,我们确定了多肽 1 与 MDM2 RING 结构域之间的关键相互作用,并设计出了多肽 2,即仍能与 MDM2 结合的多肽 1 的截短版本。结合计算和生物物理技术,我们发现多肽 2 保持了多肽 1 与 MDM2 之间的保守相互作用,并且仍能与全长的 MDM2 结合。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


