Discovery of dual-targeted molecules based on Olaparib and Rigosertib for triple-negative breast cancer with wild-type BRCA
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
PARP inhibitors (PARPis) demonstrate significant potential efficacy in the clinical treatment of BRCA-mutated triple-negative breast cancer (TNBC). However, a majority of patients with TNBC do not possess BRCA mutations, and therefore cannot benefit from PARPis. Previous studies on multi-targeted molecules derived from PARPis or disruptors of RAF-RAF pathway have offered an alternative approach to develop novel anti-TNBC agents. Hence, to broaden the application of PARP inhibitors for TNBC patients with wild-type BRCA, a series of dual-targeted molecules were constructed via integrating the key pharmacophores of Olaparib (Ola) and Rigosertib into a single entity. Subsequent studies exhibited that the resulting compounds 13a–14c obtained potential anti-proliferative activity against BRCA-defected or wild-type TNBC cells. Among them, an optimal compound 13b showed good inhibitory activity toward PARP-1, displayed approximately 34-fold higher inhibitory activity than that of Ola in MDA-MB-231 cells, and exerted multi-functional mechanisms to induce apoptosis. Moreover, 13b displayed superior antitumor efficacy (TGI, 61.3 %) than the single administration of Ola (TGI, 38.5 %), 11b (TGI, 51.8 %) or even their combined administration (TGI, 56.7 %), but did not show significant systematic toxicity. These findings suggest that 13b may serve as a potential candidate for BRCA wild-type TNBC.
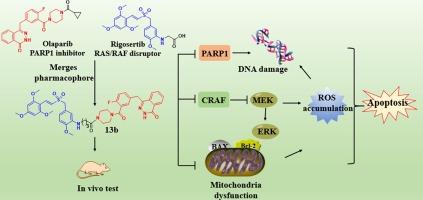
发现基于 Olaparib 和 Rigosertib 的双靶向分子,用于治疗带有野生型 BRCA 的三阴性乳腺癌。
PARP 抑制剂(PARPis)在 BRCA 基因突变的三阴性乳腺癌(TNBC)的临床治疗中显示出巨大的潜在疗效。然而,大多数 TNBC 患者没有 BRCA 突变,因此无法从 PARPis 中获益。以前对 PARPis 或 RAF-RAF 通路干扰物衍生的多靶点分子的研究为开发新型抗 TNBC 药物提供了另一种方法。因此,为了扩大 PARP 抑制剂在野生型 BRCA TNBC 患者中的应用,研究人员将 Olaparib (Ola) 和 Rigosertib 的关键药理作用整合为一个整体,构建了一系列双靶向分子。随后的研究表明,所得到的化合物 13a-14c 对 BRCA 缺陷或野生型 TNBC 细胞具有潜在的抗增殖活性。其中,最佳化合物 13b 对 PARP-1 具有良好的抑制活性,对 MDA-MB-231 细胞的抑制活性比 Ola 高约 34 倍,并具有诱导细胞凋亡的多功能机制。此外,13b 的抗肿瘤疗效(TGI,61.3%)优于奥拉(TGI,38.5%)、11b(TGI,51.8%),甚至优于它们的联合用药(TGI,56.7%),但没有表现出明显的系统毒性。这些研究结果表明,13b可作为BRCA野生型TNBC的潜在候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


