Design, synthesis and antitumor effects of lupeol quaternary phosphonium salt derivatives
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Lupeol is a natural pentacyclic triterpenoid with a wide range of biological activities. To improve the water solubility and targeting of lupeol, in the following study, we synthesized 27 lupeol derivatives in the first series by introducing lipophilic cations with lupeol as the lead compound. Through the screening of different cancer cells, we found that some of the derivatives showed better activity than cisplatin against human non-small cell lung cancer A549 cells, among which compound 6c was found to have an IC50 value of 1.83 μM and a selectivity index of 21.02 (IC50MRC-5/IC50A549) against A549 cells. To further improve the antiproliferative activity of the compounds, we replaced the ester linkage of the linker with a carbamate linkage and synthesized a second series of five lupeol derivatives which were screened for activity, among which compound 14f was found to have an IC50 value of 1.36 μM and a selectivity index of 15.60 (IC50MRC-5/IC50A549) against A549 cells. We further evaluated the bioactivity of compounds 6c and 14f and found that both compounds induced apoptosis in A549 cells, promoted an increase in intracellular reactive oxygen species and decrease in mitochondrial membrane potential, and inhibited the cell cycle in the S phase. Of the compounds, compound 14f showed stronger bioactivity than compound 6c. We then selected compound 14f for molecular-level Western blot evaluation and in vivo evaluation in the zebrafish xenograft A549 tumor cell model. Compound 14f was found to significantly downregulate Bcl-2 protein expression and upregulate Bax, Cyt C, cleaved caspase-9, and cleaved caspase-3 protein expression, and 14f was found to be able to inhibit the proliferation of A549 cells in the zebrafish xenograft model. The above results suggest that compound 14f has great potential in the development of antitumor drugs targeting mitochondria.
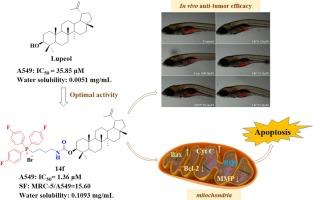
羽扇豆醇季鏻盐衍生物的设计、合成和抗肿瘤作用。
羽扇豆醇是一种天然的五环三萜类化合物,具有广泛的生物活性。为了提高羽扇豆醇的水溶性和靶向性,在接下来的研究中,我们以羽扇豆醇为先导化合物,通过引入亲脂性阳离子,合成了 27 种羽扇豆醇衍生物的第一个系列。通过对不同癌细胞的筛选,我们发现部分衍生物对人非小细胞肺癌 A549 细胞的活性优于顺铂,其中化合物 6c 对 A549 细胞的 IC50 值为 1.83 μM,选择性指数为 21.02(IC50MRC-5/IC50A549)。为了进一步提高化合物的抗增殖活性,我们用氨基甲酸酯连接取代了连接体的酯连接,合成了第二个系列的五个羽扇豆醇衍生物,并对其进行了活性筛选,发现其中化合物 14f 对 A549 细胞的 IC50 值为 1.36 μM,选择性指数为 15.60(IC50MRC-5/IC50A549)。我们进一步评估了化合物 6c 和 14f 的生物活性,发现这两种化合物都能诱导 A549 细胞凋亡,促进细胞内活性氧的增加和线粒体膜电位的降低,并抑制细胞周期进入 S 期。在这些化合物中,化合物 14f 比化合物 6c 表现出更强的生物活性。随后,我们选择化合物 14f 进行分子水平的 Western 印迹评估,并在斑马鱼异种移植 A549 肿瘤细胞模型中进行体内评估。结果发现,化合物 14f 能显著下调 Bcl-2 蛋白表达,上调 Bax、Cyt C、裂解的 caspase-9 和裂解的 caspase-3 蛋白表达,并能抑制斑马鱼异种移植 A549 细胞模型中 A549 细胞的增殖。上述结果表明,化合物 14f 在开发以线粒体为靶点的抗肿瘤药物方面具有巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


