New stilbenes from Cajanus cajan inhibit adipogenesis in 3T3-L1 adipocytes through down-regulation of PPARγ
IF 4.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Two new stilbenes, denominated Cajanotone B (CAB) and Cajanotone C (CAC), were isolated from the leaves of Cajanus cajan. In this study, the structures of CAB and CAC were unambiguously elucidated by a combination of various spectral methods. Both compounds significantly inhibited the adipogenesis in 3T3-L1 adipocytes by reducing the lipid accumulation, triglyceride content and FFA secretion. CAB and CAC also substantially inhibit the mRNA expression of HSL, ATGL, C/EBPα and PPARγ as deciphered based by RT-PCR assay. Down-regulation of PPAR is believed to be the primary mechanism underlying which CAB and CAC inhibited adipogenic differentiation because the lipid-promoting activity of PPAR agonists can be counteracted by these compounds. The molecular interaction between CAB/CAC and PPARγ was revealed with the help of molecular docking. Taken together, CAB and CAC could serve as new lead compounds with the potential to speed up the development of novel lipid-lowering and weight-control therapies.
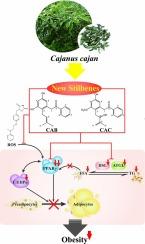
通过下调 PPARγ 抑制 3T3-L1 脂肪细胞的脂肪生成。
从 Cajanus cajan 的叶子中分离出了两种新的二苯乙烯类化合物,分别称为 Cajanotone B (CAB) 和 Cajanotone C (CAC)。本研究结合多种光谱方法,明确地阐明了 CAB 和 CAC 的结构。这两种化合物通过减少脂质积累、甘油三酯含量和 FFA 分泌,明显抑制了 3T3-L1 脂肪细胞的脂肪生成。根据 RT-PCR 分析,CAB 和 CAC 还能大幅抑制 HSL、ATGL、C/EBPα 和 PPARγ 的 mRNA 表达。下调 PPAR 被认为是 CAB 和 CAC 抑制成脂分化的主要机制,因为 PPAR 激动剂的促脂活性可被这些化合物抵消。分子对接揭示了CAB/CAC与PPARγ之间的分子相互作用。综上所述,CAB和CAC可作为新的先导化合物,有望加速新型降脂和体重控制疗法的开发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


