Mammalian D-Cysteine controls insulin secretion in the pancreas
IF 7
2区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Background
D-amino acids are being recognized as important molecules in mammals with function. This is a first identification of endogenous D-cysteine in mammalian pancreas.
Methods
Using a novel stereospecific bioluminescent assay, chiral chromatography, enzyme kinetics and a transgenic mouse model we identify endogenous D-cysteine. We elucidate its function in two mice models of type 1 diabetes (STZ and NOD), and in tests of Glucose Stimulated Insulin Secretion in isolated mouse and human islets and INS-1 832/13 cell line.
Results and Discussion
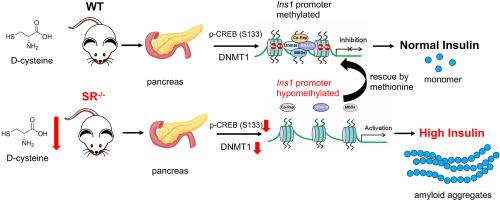
D-cysteine is synthesized by serine racemase (SR) and SR−/− mice produce 6–10 fold higher levels of insulin in the pancreas and plasma including higher glycogen and ketone bodies in the liver. The excess insulin is stored as amyloid in secretory vesicles and exosomes. In glucose stimulated insulin secretion in mouse and human islets, equimolar amount of D-cysteine showed higher inhibition of insulin secretion compared to D-serine, another closely related stereoisomer synthesized by SR. In mouse models of diabetes (Streptozotocin (STZ) and Non Obese Diabetes (NOD) and human pancreas, the diabetic state showed increased expression of D-cysteine compared to D-serine followed by increased expression of SR. SR−/− mice show decreased cAMP in the pancreas, lower DNA methyltransferase enzymatic and promoter activities followed by reduced phosphorylation of CREB (S133), resulting in decreased methylation of the Ins1 promoter. D-cysteine is efficiently metabolized by D-amino acid oxidase and transported by ASCT2 and Asc1. Dietary supplementation with methyl donors restored the high insulin levels and low DNMT enzymatic activity in SR−/− mice.
Conclusions
Our data show that endogenous D-cysteine in the mammalian pancreas is a regulator of insulin secretion.
哺乳动物的 D-半胱氨酸控制着胰腺的胰岛素分泌。
D-氨基酸被认为是哺乳动物体内具有功能的重要分子。这是首次在哺乳动物胰腺中发现内源性 D-半胱氨酸。D-半胱氨酸由丝氨酸消旋酶(SR)合成,SR-/-小鼠胰腺和血浆中的胰岛素水平高出6-10倍,肝脏中的糖原和酮体也更高。多余的胰岛素以淀粉样蛋白的形式储存在分泌囊泡和外泌体中。在葡萄糖刺激小鼠和人类胰岛分泌胰岛素的过程中,与 SR 合成的另一种密切相关的立体异构体 D-丝氨酸相比,等摩尔量的 D-半胱氨酸对胰岛素分泌的抑制作用更大。在糖尿病小鼠模型(链脲佐菌素(STZ)和非肥胖糖尿病(NOD))和人类胰腺中,与 D-丝氨酸相比,糖尿病状态下 D-半胱氨酸的表达量增加,随后 SR 的表达量增加。SR-/- 小鼠胰腺中的 cAMP 减少,DNA 甲基转移酶酶活性和启动子活性降低,CREB(S133)磷酸化减少,导致 Ins1 启动子的甲基化减少。D-半胱氨酸可被 D-氨基酸氧化酶有效代谢,并由 ASCT2 和 Asc1 转运。膳食中补充甲基供体可恢复 SR-/- 小鼠的高胰岛素水平和低 DNMT 酶活性。我们的数据表明,哺乳动物胰腺中的内源性 D-半胱氨酸是胰岛素分泌的调节剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
14.50
自引率
2.50%
发文量
219
审稿时长
43 days
期刊介绍:
Molecular Metabolism is a leading journal dedicated to sharing groundbreaking discoveries in the field of energy homeostasis and the underlying factors of metabolic disorders. These disorders include obesity, diabetes, cardiovascular disease, and cancer. Our journal focuses on publishing research driven by hypotheses and conducted to the highest standards, aiming to provide a mechanistic understanding of energy homeostasis-related behavior, physiology, and dysfunction.
We promote interdisciplinary science, covering a broad range of approaches from molecules to humans throughout the lifespan. Our goal is to contribute to transformative research in metabolism, which has the potential to revolutionize the field. By enabling progress in the prognosis, prevention, and ultimately the cure of metabolic disorders and their long-term complications, our journal seeks to better the future of health and well-being.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


