Decafluorinated and Perfluorinated Warped Nanographenes: Synthesis, Structural Analysis, and Properties
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Fluorination is a useful approach for tailoring the physicochemical properties of nanocarbon materials. However, owing to the violent reactivity of fluorination, achieving edge-perfluorination of nanographene while maintaining its original π-conjugated structure is challenging. Instead of using traditional fluorination, here, we employed a bottom-up strategy involving fluorine preinstallation and synthesized decafluorinated and perfluorinated warped nanographenes (DFWNG and PFWNG, respectively) through a 10-fold Suzuki–Miyaura coupling followed by a harsh Scholl reaction, whereby precisely edge-perfluorinated nanographene with an intact π-conjugated structure was achieved for the first time. X-ray crystallography confirmed the intact π-conjugated structure and more twisted saddle-shaped geometry of PFWNG compared to that of DFWNG. Dynamic study revealed that the 26-ring carbon framework of PFWNG is less flexible than that of DFWNG and the pristine WNG, enabling chirality resolution of PFWNG and facilitating the achievement of CD spectra at −10 °C. The edge-perfluorination of PFWNG resulted in improved solubility, lower lowest unoccupied molecular orbital, and a surface electrostatic potentials/dipole moment direction opposite those of the pristine WNG. Likely owing to its intact π-conjugated structure, PFWNG exhibits comparable electron mobility with well-known PC61BM. Furthermore, perfluorination improves thermal stability and hydrophobicity, making PFWNG suitable for use as a thermostable/hydrophobic n-type semiconductor material. In the future, this fluorination strategy can be used to synthesize other perfluorinated nanocarbon materials, such as perfluorinated graphene nanoribbons and porous nanocarbon.
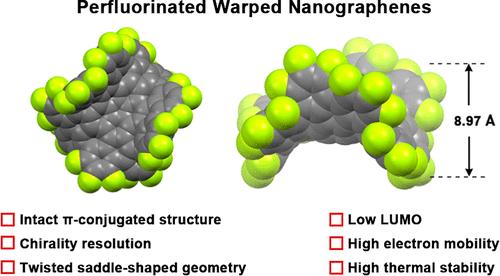
脱氟和全氟翘曲纳米石墨:合成、结构分析和特性
氟化是调整纳米碳材料物理化学特性的有效方法。然而,由于氟化反应的剧烈性,实现纳米石墨烯的边缘全氟化同时保持其原始的 π 共轭结构具有挑战性。在这里,我们没有采用传统的氟化方法,而是采用了一种涉及氟预装的自下而上的策略,通过 10 倍 Suzukii-Miyaura 偶联和苛刻的 Scholl 反应,合成了去氟化和全氟化的翘曲纳米石墨烯(分别为 DFWNG 和 PFWNG),首次实现了具有完整 π 共轭结构的精确边缘全氟化纳米石墨烯。X 射线晶体学证实,与 DFWNG 相比,PFWNG 具有完整的 π 共轭结构和更加扭曲的马鞍形几何形状。动态研究表明,与 DFWNG 和原始 WNG 相比,PFWNG 的 26 环碳框架的柔韧性较低,这使得 PFWNG 的手性解析成为可能,并有助于在 -10 °C 下获得 CD 光谱。PFWNG 的边缘全氟化改善了其溶解性,降低了最低未占用分子轨道,其表面静电势/偶极矩方向与原始 WNG 相反。可能是由于其完整的 π 共轭结构,PFWNG 的电子迁移率与众所周知的 PC61BM 相当。此外,全氟化还提高了热稳定性和疏水性,使 PFWNG 适合用作热稳定性/疏水性 n 型半导体材料。未来,这种氟化策略还可用于合成其他全氟纳米碳材料,如全氟石墨烯纳米带和多孔纳米碳。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


